I.) INTRODUCTION
Tropical forests are likely to be subject to impacts provoked by climatic change in ways that, although different from those foreseen for their temperate and boreal counterparts, are no less severe. Although global warming would cause only minimal temperature changes at equatorial latitudes, other climatic alterations expected or now in progress are likely to have major effects on forests there, with impacts that could include substantial releases of carbon. This is a function of the large areas of Brazil's forests (Figure 1). According to FAO data, 41% of tropical rainforests and 32% of all tropical forests still standing in 1990 were located in Brazil (FAO, 1994). Alterations of the water cycle are paramount among the climatic changes expected to affect these forests.
(Figure 1 here)
Changes in cloudiness and effects (both positive and negative) of CO2 enrichment may also be expected to affect forests. Climatic changes in other regions of the world can have consequences for forests in Brazil, and changes in non-Amazonian parts of Brazil can have impacts on the country's Amazonian forests. These factors include transport of nutrients to Amazonia in dust and smoke, and impacts mediated through social variables such as changes in economic growth and rates of migration to Amazonia.
In the following pages, existing knowledge is reviewed on the potential impacts of climatic change on natural forests in Brazilian Amazon. Major impacts are organized into a series of causal loop diagrams in which arrows represent the influences of different factors. These are developed for the effects on forest biomass stemming from changes in the water cycle, cloudiness, CO2 enrichment and global warming. The resulting diagrams are then combined, together with effects of climate change outside of Amazonia and social factors affecting deforestation and logging to indicate relationships between climatic changes and total forest stock.
Among the concerns that stem from possible changes in total forest stock is that it will affect the ability of these forests to supply human demands for forest products, both in Brazil and elsewhere. The Intergovernmental Panel on Climate Change (IPCC) has focussed attention on projecting these potential effects to the year 2050. It is therefore important to make the best use of the information we have available for this purpose, and to identify ways that future research and monitoring can improve the reliability of such projections. The present paper summarizes Brazil's current use levels of forest products and makes projections based on simple assumptions regarding per capita demand and population in Brazil and in the countries to which Brazil exports forest products, and the expected role of Latin America in the global trade of hardwoods. Potential alterations of this scenario that would be caused by different levels of climatic change are then explored. The low reliability of projections far into the future is recognized, a fact indicating the need for further research, including improvements in modeling the potential impacts of climatic change.
II.) CLIMATIC CHANGE PARAMETERS RELEVANT TO TROPICAL FORESTS
Water Cycle
The Amazon River is by far the world's largest in terms of water flow, with an average discharge rate of 200,000-220,000 m3 second-1 (Richey et al., 1989). The water volume is five to six times larger than the world's second largest river (the Zaïre River in Africa) and 11-12 times larger than the largest river in North America (the Mississippi-Missouri system). This great volume of water is indicative of Amazonia's importance in the water cycle. Several independent lines of evidence indicate that about 50% of the rainfall in the region originates as water recycled through the forest, including comparison of streamflow in the Amazon River with water falling as rainfall throughout the drainage basin (Leopoldo et al., 1982; Villa Nova et al., 1976), evidence from isotope ratios in water vapor (Marques et al., 1977; Salati et al., 1979), and forest heat balance (Molion, 1975) (see review in Fearnside, 1990a). Because half of the rainfall is recycled, the water volume involved is the same as that seen flowing in the Amazon River. Even small percentage alterations in evapotranspiration would therefore affect very large fluxes of water vapor. Consequent reductions in rainfall potentially affect not only Amazonia but also Brazil's central-south region where most of the country's agriculture and silviculture are located (Eagleson, 1986; Salati and Vose, 1984).
The great majority of deforestation in Brazilian Amazonia is followed by conversion of land to cattle pasture, either immediately or after one to two years of use under annual crops (Fearnside, 1987a). Present behavior patterns, if continued unchanged to equilibrium, imply a future landscape composed of 44.9% secondary succession derived from pasture, 43.8% productive pasture, 5.2% degraded pasture, 4.0% farmland, and 2.0% secondary succession derived from agriculture (Fearnside, 1995a).
Since evapotranspiration is proportional to leaf area, the water recycled through forest is much greater than that recycled through pasture, especially in the dry season when pasture is dry while forest remains evergreen. This is aggravated by the much higher runoff under pasture. Increases in runoff by one order of magnitude have been measured near Manaus (Amazonas), Altamira (Pará) and Ouro Preto do Oeste (Rondônia) (see Fearnside, 1989a). Soil under pasture quickly becomes highly compacted, inhibiting infiltration of rainwater into the soil (Dantas, 1979; Schubart et al., 1976). Rain falling on compacted soil runs off quickly, becoming unavailable for later release to the atmosphere through transpiration. Pasture has much less leaf area than forest (McWilliam et al., 1993), and its shallower root system prevents it from transpiring during periods of drought (Nepstad et al., 1995). Secondary forests also have root systems that are shallow relative to those of primary forest (Nepstad et al., 1995).
The potential damage of lowered rainfall for the remaining natural ecosystems is indicated by the seasonal and spatial patterns in water vapor sources found by Salati et al. (1978, 1979). The importance of recycled water is greatest in the dry season, and increases as one moves farther from the Atlantic Ocean. This means that in Rondônia and Acre, the proportion of rainfall derived from forest could be much higher than the roughly 50% found in the Belém‑Manaus transect.
The greater dependence in the dry season means that conversion to pasture would cause this period to become longer and more severe, a change that could have severe repercussions on the forest even if total annual precipitation were to remain unchanged. Many rainforest trees are already at their limits of tolerance for drought stress. In patches of forest isolated by cattle pasture in the INPA/Smithsonian Institution 'Biological Dynamics of Forest Fragments' project near Manaus, trees on the edges of forest patches die at a much greater rate than do those in continuous forest (Lovejoy et al., 1984; Rankin-de-Merona et al., 1990). Since many trees die while still standing, rather than being toppled by wind, dry conditions (particularly in the air) near the reserve edges are a likely explanation for the mortality (Fearnside, 1985a). Soil water may act to partially counterbalance the effect of drier air: as trees die, soil water in the gaps they leave normally increases since the roots are gone that would have removed water from the soil.
Drier microclimatic conditions have been found at forest edges (Kapos, 1989). Increased water stress, as indicated by altered 13C in plant leaves, extends 60 m into the forest from an edge (Kapos et al., 1993). The potential for edge effects to greatly magnify the impact of Amazonian deforestation has been shown by Skole and Tucker (1993) in a calculation assuming an arbitrary distance of 1 km of disturbance from all Amazonian clearings, indicating a 'disturbed' area of 34.1 X 106 ha, or 148% of the area that these authors found had been cleared by 1988 (but see Fearnside, 1993a for discussion of the deforestation estimate). While microclimatic effects 1 km from an edge are unlikely and have not been demonstrated anywhere, improved human access provided by clearing often leads to increased hunting and to removal of the most valuable timber at such distances.
As with many impacts of climatic change, the greatest effects are likely to occur during occasional 'extreme' events rather than gradually from year to year. Precipitation in Amazonia is characterized by tremendous variability from one year to the next, even in the absence of massive deforestation (Fearnside, 1984). Were the forest's contribution to dry season rainfall to decrease, the result would be to increase the probability of droughts more severe than those experienced in the centuries or millennia over which the present forest became established. A very severe drought once in, say, 20 to 50 years would kill many trees of susceptible species. The result would be replacement of the tropical moist forest with more drought‑tolerant forms of scrubby, open vegetation resembling the cerrado (scrub savanna) of central Brazil (Fearnside, 1979).
Drying could activate a positive feedback process, whereby reduced evapotranspiration (caused by deforestation) leads to more severe dry periods, causing death of trees, thereby further reducing evapotranspiration, increasing water stress, and leading to still more mortality (Fearnside, 1985a). Simulations that incorporate this feedback indicate retraction of Amazonian forest and replacement of substantial areas with cerrado vegetation (Shukla et al., 1990).
Severe droughts provoked by deforestation could lead to rapid erosion of the remainder of the forest once a substantial portion of the region had been converted to pasture. In Amazonia at present, burning is almost entirely restricted to areas where trees have been felled and allowed to dry before being set alight. Fire stops burning when it reaches the edge of a clearing rather than continuing into unfelled forest. This lucky situation need not necessarily continue unchanged. In forested areas that have been disturbed by logging along the Belém‑Brasília Highway, fires from neighboring pastures have already been observed to continue substantial distances into the standing forest (Uhl and Buschbacher, 1985). During 1982‑83 (an unusually dry year because of the El Niño/Southern Oscillation phenomenon) approximately 45,000 km2 of tropical forest on the island of Borneo burned when fires escaped from shifting cultivators' fields. At least 8,000 of the 35,000 km2 of this area in the Indonesian province of East Kalimantan was primary forest, while 12,000 km2 was selectively logged forest (Malingreau et al., 1985). Devastation would be catastrophic should fires such as this occur in Amazonia during one of the droughts aggravated by drying from deforestation. Archeological evidence suggests that catastrophic fires have occurred in Amazonia at the times of major El Niño events four times over the past 2000 years: 1500, 1000, 700 and 400 B.P. (Meggers, 1994). Human action could now turn less-intense El Niño events, which are much more frequent than major ones, into major catastrophes.
Droughts lead to increases in the area and completeness of burning in clearings in Amazonia, contributing to smoke and dust that function as a source of wind-borne nutrients to the surrounding forest. Soil nutrients and light are among the factors that limit growth, recruitment and mortality of trees.
Mortality is also affected by insects, which are in turn influenced by droughts. More severe dry seasons could result in opposing influences on the frequency of insect outbreaks. Fewer outbreaks would be expected to the extent that a severe dry season in tropical areas serves as a form of 'free' insect control similar to that provided by the temperate-zone winter (Janzen, 1970, 1973). On the other hand, moisture- or nutrient-stressed crops (or trees) are more likely to be attacked by insect pests (Cammell and Knight, 1992). Climatic change will expose natural forests, as well as plantations and other ecosystems, to water stress, making plants more susceptible to insect and disease attack.
One of the major impacts of climatic change on forestry is the possibility of increased mortality of natural forest trees as a result of water stress. This would negatively affect timber production by: 1) hastening retreat of forest boundaries, 2) reducing biomass of forests (including the large trees most valuable for timber), and 3) slowing regeneration of biomass to replace that harvested or killed in logging operations.
Links between droughts and biomass of natural forests are summarized in Figure 2. In causal loop diagrams such as this, the sign indicated for each arrow represents the direction of change that would be expected in the quantity at the head of the arrow given an increase in the quantity at the tail of the arrow.
(Figure 2 here)
Cloudiness
Prediction of how clouds will change is currently a weak point in climate models. It is possible that increased evaporation due to temperature rises over oceans could increase cloudiness in eastern Amazonia, while at the same time, possible reductions in evapotranspiration due to deforestation could reduce cloudiness in western Amazonia. Insolation is one of the determinants of primary productivity and, despite the reputation of the tropics for a superabundance of sunlight, can limit growth. Cloud cover is greatest in the State of Amapá, located in the northeast corner of Brazilian Amazonia between French Guiana and the mouth of the Amazon River. Cloudiness in this part of the region is so persistent that in many years none of the 40 annual passes of LANDSAT satellites at each point on the ground provides an image usable for estimates of deforestation (see Fearnside, 1990b).
At the Jari estate on the Pará/Amapá border, clouds limited yields of irrigated rice (C. Wang, personal communication, 1979; see Fearnside and Rankin, 1980). Changes in cloudiness might affect yields of the 100,000 ha of silvicultural plantations at Jari, and 70,000 ha at AMCEL (in central Amapá), as well as have effects on native forest in the area. Because Amapá is near the Atlantic Ocean and receives most of its water vapor from that source (Salati et al., 1978), any climatic change-induced alteration of cloudiness in Amapá is most likely to be an increase. The presumable consequence of this would be decreases in primary productivity of plantations and natural forests.
Links between cloudiness and forest biomass are summarized in Figure 3. Light, as one of the regulators of growth, recruitment and mortality, is limited both by cloudiness and by competition from neighboring trees (a function of forest biomass).
(Figure 3 here)
CO2 Enrichment
Carbon dioxide increase in the atmosphere would lead to increased productivity anywhere where growth is not limited by other factors such as soil fertility, insolation and water availability. It is important to realize that a single limiting factor does not govern growth of a forest as a whole, or even growth at all times of a single individual plant. Growth rates of some plants might therefore increase in the future, or even be faster today than it would be if the earth still had its pre-industrial atmosphere. Responses of individual tree species, however, provide poor indications of the changes that may result at the community or ecosystem levels (Bazzaz, 1990: 190).
Phillips and Gentry (1994) found a consistent worldwide pattern of increasing turnover of individuals at 22 tropical forest sites with two or more measurements during the 1950-1993 period. One possible mechanism they suggest for this phenomenon is differential stimulation of growth of tree-killing vines as a result of CO2 enrichment (the other mechanism is increase in periods of stress from droughts and storms caused by global climatic change). Phillips and Gentry (1994) found increases in vine density at five of the six tropical forest sites with data on this life form. Vine growth in general has been found to be greatly stimulated by increased CO2 under laboratory conditions (Condon et al., 1992). The increased mortality in trees strangled by vines would make 'undisturbed' tropical forests a major source of CO2 release.
The opposite effect of CO2 enrichment is also possible: stimulated growth of at least some forest trees, serving to remove carbon from the atmosphere (Lugo and Brown, 1992). Because most tropical forest trees face critical limitations on their growth from lack of nutrients and from competition with their neighbors for light, water and other resources, CO2 enrichment is not thought likely to result in a generalized increase in tree growth (e.g. Liss and Crane, 1983). However, a substantial uptake is indicated by the one existing direct measurement of CO2 flux with more than a few hours duration. A measurement over undisturbed forest in Rondônia for a period of 44 days, when extrapolated to a full year based on local weather data and to Amazonia as a whole as a function of forest area, indicates an annual net uptake of 500 X 106 t C (Grace et al., 1995). The proportional uptake in the 4 X 106 km2 of forests in the Brazilian portion of Amazonia would be 400 X 106 t C. The possibility of such a large flux indicates the importance of obtaining measurements at additional locations. The high gross primary productivity and large area of tropical forests mean that even a small shift in the balance between growth and respiration in these ecosystems would have major implications for the global carbon cycle.
The links if CO2 fertilization to forest biomass are summarized in Figure 4. While growth and recruitment are stimulated, so is tree mortality (i.e. through the effect on vines). As with other climatic factors, competition for light is a key means by which the effect of increased forest biomass feeds back on tree growth, recruitment and mortality.
(Figure 4 here)
Global Warming
While temperature changes from global warming are expected to be modest in the tropics as compared to temperate regions, it is important to realize that each degree of temperature alteration in a tropical environment may be 'perceived' by forest species there as a 'greater' change than would be the case for the same temperature shift in a temperate forest. This is because species that have evolved in more temperature-stable environments have narrower ranges of tolerance, and has been suggested by Janzen (1967) as a reason why "mountain passes are higher in the tropics"--that is, why a given elevational barrier in the tropics prevents the crossing of more species than would the same barrier in temperate zones. The direct effects of global warming on ecosystems at relatively low latitudes, if not at the equator itself, may therefore be greater than the small predicted temperature alterations at these sites might lead one to believe.
Direct effects of global warming through temperature change are likely to be less pronounced than effects that temperature can have through its influence on other climatic parameters such as rainfall. Precipitation reduction in Amazonia during June-July-August (dry season for most of the region) would be 1 mm day-1 "around the time of CO2 doubling" (after 2100), according to United Kingdom Meterological Office global circulation model (GCM) used by the IPCC 1992 supplementary report (Gates et al., 1992: 200). Considering 30-year monthly averages for precipitation at 11 meteorological stations in Amazonia reported by da Mota (1981: 297), 1 mm day-1 represents a 34% decline in dry season rainfall.
The changes expected to result from doubling the present level of global warming impact from greenhouse gases are thought to lead to a 10-15% expansion of the area suitable for tropical forests as equilibrium vegetation types (Solomon et al., 1993). For tropical rainforest, the suitable area would expand 7 to 40%, depending on the GCM employed in estimating the future distribution of climatic zones. The GCM studies used by Solomon et al., (1993) assess effects of doubled greenhouse gases (i.e., CO2-equivalence), both through direct effects of temperature and through temperature-driven alteration of precipitation regimes (but not rainfall changes provoked by deforestation). These results are indicators of potential for forest expansion, and are not intended to reflect expected landscapes in the future: they do not include the influence of human populations in converting to other uses land that is climatically suitable for tropical forests.
One model that includes both climate-induced and human changes to the year 2050 points to decreases in forest areas by about 23% worldwide and by about 5% in Latin America (Zuidema et al., 1994). The deforestation estimates used in these calculations are based on the areas needed to satisfy expected demands for agricultural products and, in the case of Africa and South Asia, for fuelwood. In the case of Brazil, deforestation is likely to exceed these forecasts, as much of the forest clearing in that country stems from motivations other than consumption of agricultural products (Fearnside, 1987a, 1993b; Hecht, 1993; Hecht et al., 1988; Reis and Margulis, 1991). In any case, the combination of forces driving deforestation throughout the tropics makes it unlikely that tropical forests will be permitted to expand to occupy the increased areas made climatically suitable for them by global warming.
The principal links of global warming to forest biomass are shown in Figure 5. These include both direct effects of temperature and precipitation changes in the region and the effects of extra-regional transport of dust and smoke (to be discussed in the next section).
(Figure 5 here)
III.) IMPACTS FROM CLIMATIC CHANGE IN OTHER REGIONS
Climatic changes in distant places can have impacts on tropical forests. One mechanism is by stimulating migration to tropical forest areas. Successive waves of migrants to Amazonia from semiarid northeastern Brazil have resulted from recurrent droughts in the Northeast. While droughts in northeastern Brazil are tied to pressure fluctuations in the Northern Hemisphere and over the South Atlantic (Buchmann, 1981), they are probably made more severe by removal of vegetation in the areas affected (Fearnside, 1979), and their impacts on the human population are more severe due to the dense population relative to the productive capacity of the land (Fearnside, 1986a). The São Francisco River, source of much of the existing irrigation water in northeastern Brazil, is expected to have reduced flow as a result of global warming, according to some GCM results (see Revelle, 1982). However, GCM results for rainfall at low latitudes are sufficiently inconsistent that, pending the availability of better models, few researchers have ventured to calculate the potential impact of precipitation changes on agricultural production (Parry, 1992).
Should droughts in Northeast Brazil be aggravated by global warming, increased migration to Amazonia would be a logical consequence. On the other hand, should increased evaporation over the Atlantic Ocean lead to increased precipitation, the situation would be helped instead. In other parts of the world, such as the Sahel region of Africa, drought-motivated migration may become an increasingly severe source of pressure on remaining tropical forests adjacent to drought-prone areas (Myers, 1993).
In many semiarid portions of the tropics, reduced firewood regrowth can be expected to result from increased severity of droughts (as well as from increased frequency of cutting by growing human populations). Firewood supply is the principal role of the forest sector in the human economy in these areas. It should be noted, however, that credit for avoided deforestation by firewood-starved migrants who might move from semiarid to tropical areas (as from Northeast Brazil to Amazonia) cannot legitimately be claimed as a credit for firewood plantations and/or dry forest management schemes proposed as global warming response options in semiarid areas (as has sometimes been done). Lack of firewood, while it may contribute something to individual decisions to migrate, can hardly be assumed to be the dominant factor in the Brazilian Northeast.
Inducement of migration by global warming is only one potential consequence of climatic changes in non-Amazonian parts of Brazil. Many impacts of climatic change are likely to harm Brazil's overall economy. Less economic growth implies reduction of available funds for investment in ranching and other activities, which would act to slow Amazonian deforestation. A slowdown of this type occurred over the 1987-1991 period in response to Brazil's economic recession.
Other climatic events can provoke migrations to tropical forest areas. Destruction of coffee plantations in the State of Paraná by frosts in 1975 marked the beginning of a wave of migration to Amazonia from that southern Brazil state. Ironically, many of those who left Paraná were originally from Northeast Brazil, but had left that densely populated region during one of the frequent droughts there.
Another climatic event with far-reaching effects for tropical forests is the El Niño/Southern Oscillation. Among other effects around the world, in the 1970s this recurrent event contributed a coup-de-grace for the anchovy fishery off the coast of Peru. The anchovy population had already been depleted through prolonged overfishing. The end of the commercial fishery contributed to use of soybeans as a substitute for fish meal in animal feeds used in North America and Europe (M. Glantz, personal communication, 1985). The resulting increase in soybean prices led to rapid expansion of mechanized soybean cultivation in Paraná. Many small farmers displaced by replacement of labor-intensive crops (such as coffee) in Paraná migrated to Rondônia, where they were a key factor in one of the world's most rapid explosions of tropical deforestation activity (Fearnside, 1986b, 1987b).
African dust transported across the Atlantic Ocean by winds may be supplying significant amounts of phosphorus and calcium to Amazonia (Swap et al., 1992). Amazonian soils are very poor in these elements (Fearnside, 1986a). The amount of dust is increased by overgrazing and other land-use and land-management changes in Africa and by a climate characterized by increasingly severe drought events. Nutrients are also contributed by smoke and ash particles from burning in savannas, possibly including those in Africa (Talbot et al., 1990). The extent to which these nutrient sources could increase growth of Amazonian forests is not known. Increases undoubtedly differ by tree species, thereby altering forest composition. Burning is affected by climate, as well as by the size and behavior of the human population.
Figure 6 presents a causal loop diagram uniting the four climatic change parameters for which connections with forest biomass were diagrammed earlier (droughts, cloudiness, CO2 fertilization and global warming), together with the effects of climatic changes outside of Amazonia and social factors affecting deforestation and logging. From this, the effects on forest biomass and forest area can be combined to reflect changes in the total forest stock, the measure most closely related to the future availability of wood and other products from natural forests.
(Figure 6 here)
IV.) CURRENT AND PROJECTED USE LEVELS OF FOREST PRODUCTS
Current Use Levels
Wood consumption and supply need to be separated by type of wood if potential contributions of native forest logging and plantations are to be understood. Most wood produced by plantations in Brazil is from short-rotation systems that yield wood appropriate for pulp, firewood or charcoal, but not for sawnwood or wood-based panels. The distinction is very important, as it means that plantation output rarely can substitute for native forest logging, and that environmental benefits of avoided logging and associated deforestation cannot be legitimately claimed for plantation promotion programs.
The reverse is also true: logging cannot substitute for plantations. Pulpwood produced in short-rotation plantations cannot, for the most part, be supplied by logging Amazonian forest. For example, Jari used 80 native forest species for pulpwood in 1983 (Fearnside and Rankin, 1985: 125), but reduced this to 40 species in 1986 (Fearnside, 1988: 18). The upland forest contributions are mostly for mixture with Gmelina (to which they contribute a volume supplement of only 5%) rather than the Eucalyptus species that appear more likely to dominate pulpwood plantations in Amazonia. No native species are mixed with Pinus caribaea pulpwood (Fearnside, 1988: 17).
The information needed is different from that usually reported in forestry statistics, which provides information on 'industrial roundwood' production, consumption and exports. Industrial roundwood includes logs (roundwood), sawnwood, wood-based panels and pulpwood. It does not include firewood or charcoal. From the point of view of supply, it is important to separate pulpwood from other categories of industrial roundwood, because pulpwood and other categories are produced by different systems.
Non-timber forest products, such as rubber and Brazil nuts, are also important. Rubber produced from native forest extraction is not competitive with imports from Southeast Asia (in the absence of protectionist trade barriers). In 1987 rubber harvested from native forests totaled 26.4 X 106 t of latex, while Brazil nuts totaled 36.2 X 106 t (Brazil, IBGE, 1989: 332). A variety of other extractive products is harvested for fibers, gums and resins, essential oils, tannins, foods and medicinal plants. There is also some illegal trade in live animals and in animal skins.
Brazil's system of extractive reserves represents a valuable initiative to enlist the population of rubber tappers and Brazil nut gatherers in maintaining the forest resource (Allegretti, 1990). The flow of products from the reserves has been increasing as the number of reserves has grown since the first was established in 1988. The economic situation of the reserves has been dealt a severe blow, however, by the continued decline in rubber prices--a combined result of reduced subsidies and falling prices for rubber in international commodities markets. It is essential that the basic justification of the extractive reserve concept be recognized in terms of maintaining the environmental services of the forest rather than as a source of supply of non-timber forest products (Fearnside, 1989b).
The value of Amazonian forests for 'ecological' uses such as biodiversity maintenance, carbon storage and water vapor supply overshadows by far both current and potential revenue from both timber and non-wood products. It would be better to base forest protection on this real value of the forest, rather than on its potential to be managed as a source of wood or other commodities. At present, however, institutional arrangements are completely lacking to turn many of these forms of value, such as environmental services, into a means of supporting the region's human population.
'Social' values, such as aesthetics and environmental ethics, represent a kind of forest product that must have its proper place defined in decisions regarding the forest. The social value of forest is an important part of the lives of traditional forest peoples such as rubber tappers and Amerindians. These values, however, play a minimal role among those who do most of the forest clearing in Brazilian Amazonia: ranchers (responsible for about 70% of the clearing) and small farmer immigrants (responsible for about 30%: see Fearnside, 1993b). Social values are largely 'consumed' outside Amazonia, either in Brazil's urban south or abroad. The importance of aesthetics is most likely to be felt in Amazonia by means of its motivation of international money flows, rather than through direct influence on the decisions of anyone whose hand is on a chainsaw. Occasional proposals to slow deforestation through environmental education for 'consciousness raising' among rural Amazonians are not likely to meet with success.
Projected Use Levels
The flows of tropical forest products are likely to change dramatically over the coming decades as forests in Africa and especially in Southeast Asia are decimated by deforestation and overlogging, quite apart from effects of climate-induced changes. The international timber trade is likely to focus increasingly on the remaining forests in Latin America as current sources of supply in Asia are exhausted. The larger remaining forest areas in Latin America, particularly Brazil, would continue to supply markets for some time, but these forests too are finite and would eventually come to an end. Unless dramatic changes can be brought about in the way in which financial decisions are made by logging enterprises, tropical forest management has virtually no hope of altering this outcome (Fearnside, 1989c). The hastened demise of tropical forests provoked by climatic change would lead to an earlier end of timber supplies from these sources.
Some simple assumptions can be used to have a rough idea of future wood product supplies in Brazil in the absence of impacts from climatic change. Deforestation is assumed to proceed through 2030 following the pattern projected by Reis and Margulis (1991), and for the 2030-2050 period by continuing the trend expected by these authors for the 2000-2030 period. Reis and Margulis (1991) calculated annual deforestation rates using a regression based on a sample of 165 municípios (counties), using data on human population, cattle population, length of roads, distance to the state capital, percentage of area in crops, and volume of timber logged. Município-level deforestation rates were based on various available LANDSAT studies providing data for Acre in 1983, Amazonas in 1987, western Mato Grosso in 1986, Rondônia in 1987, Roraima in 1983, and Pará in 1986 (see Fearnside, 1990b for a review of these studies). Most independent variables refer to 1985, based on the agricultural census of the Brazilian Institute for Geography and Statistics (IBGE). The exceptions are logging activity, which was based on IBGE's extractive plant production tables for the 1982-1985 period, and road length, which was measured from National Highway Department (DNER) maps for 1985 or 1986. The populations of the municípios were from the preliminary IBGE estimate for 1985.
Reis and Margulis (1991) used the regression developed for 1985 to project deforestation over the 1985-2000 period, assuming a 6.2% year-1 growth of the gross regional product, which is assumed to be a constant proportion of a 5.0% year-1 growth of the gross national product (GNP), based on a regional input-output model by Castro (1989: 303). The projection of deforestation for the 1985-2000 period assumes annual growth rates in Amazonia of 3.2% for population, 6.0% for cropped area, 8.0% for the cattle herd, and 7.5% for timber harvesting. Roadbuilding over the 1985-2000 period is assumed to be determined by external political decisions to complete by the year 2000 all planned roads listed (at the state level) in Brazil's 1989 statistical yearbook (Brazil, IBGE, 1989).
Reis and Margulis (1991) made a separate projection of deforestation over the 1990-2030 period. For the 2030 estimate, Reis and Margulis (1991) assumed the following annual growth rates applied to the 1990-2030 period in Amazonia: 2.0% for population, 5.0% for cropped area, 7.0% for the cattle herd, 6.5% for timber harvesting, and 2.1% for road length.
The Reis and Margulis (1991) projections of deforested area in 2000 and 2030 are used to construct a scenario for future changes in forest area. Remaining forest areas are interpolated between 1990 and 2000 and between 2000 and 2030, and extrapolated to 2050, making the conservative assumption of a linear trend at the average 2000-2030 rate of 36 X 103 km2 year-1. Under these assumptions, forest area in the Brazilian Legal Amazon in 2050 would be reduced to 44% of the 3.6 X 106 km2 present in 1990 (excluding the area flooded by hydroelectric dams).
International exports of tropical hardwoods from Brazil are assumed to follow the pattern simulated by Grainger (1990). No attempt was made to link deforestation rate to projected levels of logging activity (i.e., the deforestation scenario maintains the assumptions of Reis and Margulis regarding logging). The present calculation assumes that logging for sawlog-equivalent timber (the vast majority of the total extracted) was being done at an intensity of 20 m3 ha-1 in 1990. For comparison, FAO (1994) reports an average logging intensity for closed broadleaf forests in Brazil of only 6 m3 ha-1 per time logged, with the possibility of a given area being re-logged for an unspecified number of times at the same 6 m3 ha-1. In reality, the intensity of logging varies tremendously, with some forest being felled for agriculture and ranching without any timber removal, while other areas have as much as 75 m3 ha-1 removed. Forests near Paragominas, Pará, are logged at 50 m3 ha-1 (Uhl and Vieira, 1989).
Light-intensity logging for very valuable species such as mahogany (Swietenia macrophylla) takes place over tremendous areas, and larger volumes of less-valuable timber are often removed later as the frontier draws nearer (Veríssimo et al., 1995). The calculation makes the optimistic assumption that logged-over forest is always deforested before unlogged forest.
The wood export volumes assumed follow the pattern simulated by Grainger (1990) through 2005 for Latin America, but have been scaled to volumes reported by FAO (1993) for 1991. This may be making the projection overly optimistic, as the FAO (1993) report indicates total exports from South America in 1991 of only at about one-third the amount that Grainger (1990) had forecast for 'Latin America.' Underreporting to FAO may, of course, be an explanation. The period through 2005 covers the expected shift from Asia and Africa to Latin America of the dominant role in supplying the tropical timber trade. The total volume of exports from Latin America (and consequently from Brazil) increases greatly over this period.
The percentage of Latin American tropical hardwood exports over the 1990-2005 period that come from Brazil is assumed to be in proportion to Brazil's share of sawlog-equivalent products exported from South America in 1991 as reported by FAO (1993) (note, South America accounts for the great majority of exports from Latin America; note also: FAO, unlike other UN agencies, does not consider 'South America' to include the three Guianas). The exported wood from logging is apportioned into sawnwood, roundwood (logs), and wood-based panels in the same proportions as 1991 Brazilian exports reported by FAO (1993). Exports of charcoal and pig iron (which must be considered as a forestry product in the Brazilian context) receive some input from logging (assumed to be from forest exploitation at 75 m3 ha-1 to provide an initial (1990) value of 100 X 106 m3 year-1). This is to reflect forest biomass use for making charcoal for pig iron smelting in the Grande Carajás area (an activity which could require 100-150 X 103 ha year-1 if all planned smelters were implanted: Anderson, 1990; Fearnside, 1989d). Forest biomass is currently used for firewood in powerplants at Jari (Monte Dourado, Pará), Manacapuru (Amazonas) and Ariquemes (Rondônia).
After the year 2005, the volume of hardwood exports is assumed to increase in proportion to the increase in population of non-tropical countries of the world, using population projections adopted by the World Bank in 1992. Presumably populations of tropical countries, which would be increasing at a rate faster than that in non-tropical countries over this period, would not have sufficient funds to import Brazilian wood to a significant extent. The World Bank's projection for non-tropical countries foresees a decline in their population over the 2020-2050 period from 3.8 to 3.6 billion, meaning that Brazilian wood exports ratioed to this population would decline similarly. Grainger's (1990) model of the world market for tropical timber projects a decline in exports from Latin America after 2005 as a result of increasing diversion of production to satisfy local demand, and possibly also as a result of exhaustion of Latin American timber stocks. Since these factors are included in the present calculation, use of Grainger's projections after 2005 would duplicate these effects, with the result being an overly optimistic picture of restraint, leaving substantial timber stocks unexploited in Amazonia.
Domestic consumption of all wood products is assumed to increase in proportion to the national population, assuming that Brazil's annual population growth rate continues to decline at the same rate observed over the last two census periods (annual rate of population growth dropping by 0.2 percentage points each decade). The population would reach 363 million by 2050 under this assumption.
Industrial roundwood export and consumption from domestic sources is shown in Figure 7, and domestic consumption of sawlogs is shown in Figure 8. Pulp and firewood consumption is projected in Figure 9.
(Figures 7, 8 and 9 here)
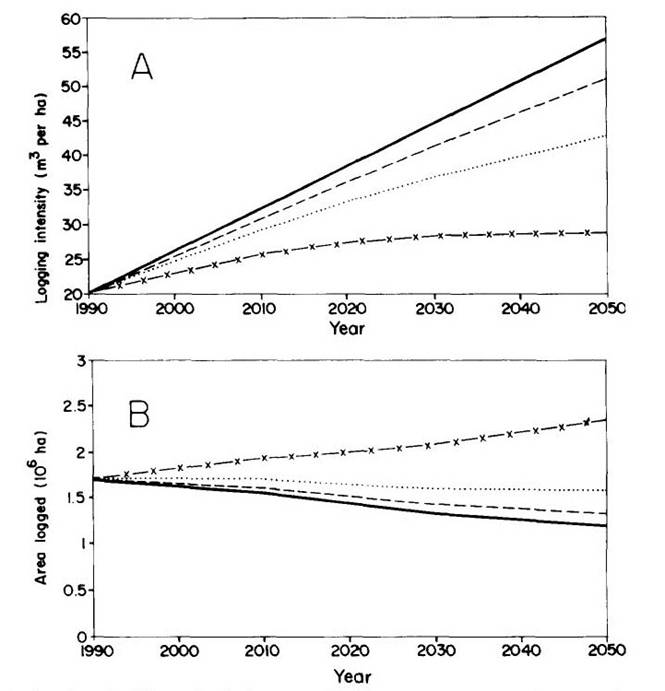
We currently have insufficient knowledge to make quantitative predictions of either the extent to which different climatic parameters are likely to change in Amazonia or what the magnitude of the forest's response will be. However, we do know that many of the changes would lead to forest degradation. One can obtain useful insights from calculations under a series of scenarios that assume different rates of biomass loss. Scenarios with loss in standing forest by the year 2050 totaling from zero to 50% of the biomass can be assumed to bracket the possible impacts.
In the absence of climatic change and given the assumptions regarding the level of demand for native forest timber (which may be optimistically low), the area of native forest is more than sufficient to maintain supply through 2050. The intensity of logging increases over time as more species are accepted by the market (the percentage of the biomass marketable being assumed to change linearly to double by 2050 for sawlog-equivalent timber; no change is assumed for firewood or charcoal wood from logging). Under the climatic change scenarios, the thinning of forest biomass makes it necessary to harvest a larger area each year to supply the demand (Figure 10). The assumed demand levels continue to be met, however, through 2050 even under the most severe climatic change scenario.
(Figure 10 here)
V.) RESEARCH AND MONITORING NEEDS
Biological processes
The present state of knowledge on interrelations of climate and tropical forests is far from adequate. Current inadequacies of the information base should never be used to justify delaying action to slow deforestation and other forms of environmental degradation. The current state of knowledge is more than sufficient to identify major problems and needed policy changes.
Areas of knowledge in need of reinforcement include gathering, manipulation and interpretation of data on forest area changes, associated population and economic factors, and physical processes in forests themselves. To begin with, physical processes need to be better understood. In areas along the forest-cerrado border, fire frequency and effects need to be quantified. Fire frequency can be detected relatively inexpensively by remote sensing using the thermal infrared band of the NOAA Advanced Very High Resolution Radiometer (AVHRR). Although the number of fires has been counted for several years using this technique (e.g. Setzer and Pereira, 1991), the frequency of fire recurrence at any given point has not been determined. AVHRR has a resolution at best of 1.1 km, which means that field studies (probably including aerial photography) are necessary if 'fire pixels' are to be interpreted in terms of burning at specific points on the ground. Burning in the cerrado that borders Amazonian forest to the south has increased in frequency over the historical past. This is believed to create an unfavorable nutrient balance for this ecosystem (Coutinho, 1982, 1990).
Fire in adjacent non-forest areas, including burning in cattle pastures in clearings within the forest, can have significant impacts on forests. Fire invasion of standing forest extends the impact of deforestation (Uhl and Buschbacher, 1985; Uhl and Kauffman, 1990). Fires could play a similar role in hastening the retreat of the forest boundary with the cerrado. Further research on the impact of fire is indicated.
Improving estimates of forest biomass is a high priority. Biomass is directly related to carbon storage, and therefore to global warming impacts of forest loss and benefits of avoiding such loss (and consequently the relative attractiveness of deforestation reduction versus other forestry response options such as plantations). A prolonged controversy has surrounded the question of biomass estimation (e.g. Brown and Lugo, 1984, 1992a,b,c; Brown et al., 1989; Lugo and Brown, 1986; Fearnside, 1985b, 1986c, 1992a,b, 1993c). The quantity and quality of forest inventory and other data available have steadily improved, as has interpretation of what these data mean in terms of biomass and emissions (see review in Fearnside et al., 1993). Average biomass of forests in Brazil is substantially higher than was previously thought. The average total biomass of original forest of 464 t ha-1 (Fearnside, 1995b) is 32-200% higher than biomass estimates adopted in deriving deforestation emissions values used in the IPCC's 1990 report (Watson et al., 1990: 11) and 1992 supplement (Watson et al., 1992: 33).
Leaf area, root depth, and tolerance to water stress are characteristics of tropical forest trees that need study. These characteristics are important to the role of trees in the water cycle at present and under an altered climate. The potential effects of longer dry seasons need to be forecast with greater confidence, as this will determine future vegetation cover over vast areas, with consequent potential for tremendous releases of greenhouse gases.
Related to the question of water tolerance is the need to monitor turnover (mortality and regeneration) of tropical forest trees. The worldwide increase in turnover rates found by Phillips and Gentry (1994) has major potential for release of carbon now stored in forests. Unfortunately, Brazil's Amazonian forests are poorly studied in this regard relative to other tropical forests, with only 4 of 40 sites with at least one measurement located in Brazil, and none of the 22 sites with two or more measurements. Average turnover increased from 1.4% year‑1 to 2.2% year-1 over the 1980-1990 period. It is important to realize that 'turnover' as reported by Phillips and Gentry (1994) refers to turnover of individuals, not biomass. The corresponding percentage for biomass would be much higher because mortality is distributed across all size classes, including large trees, whereas recruitment is necessarily restricted to entry from the bottom of the diameter range considered in each survey.
Deforestation
Research and monitoring need to be reinforced in areas that can be expected to yield the most benefits in reducing environmental impacts, including climatic change. A regular program for deforestation monitoring by satellites is necessary, striking a reasonable balance between cost of coverage and detail of information. LANDSAT-TM 1:250,000 scale imagery has proved to be a viable choice in Brazil (Fearnside, 1993b; Fearnside et al., 1990). The Brazilian data have shown the great spatial heterogeneity of clearing and consequent importance of 'wall-to-wall' coverage for assessing deforestation (see Fearnside, 1993b). While Brazil's National Institute for Space Research (INPE) has obtained and interpreted images for all 229 LANDSAT-5 scenes needed to cover the 5 X 106 km2 Legal Amazon region, limited resources have forced FAO's monitoring program to use a small sampling of scenes in deriving land-use change data for much of the rest of the tropics (including parts of Brazil outside the Legal Amazon, and cerrado vegetation within the Legal Amazon). Sufficient resources must be invested to obtain regular coverage of all tropical forest areas in the world.
Interpretation of imagery for deforestation in Brazil has so far been limited to producing tabulations by state, although separation into municípios should become available soon. Efforts to superimpose spatially referenced deforestation information on vegetation maps are also underway. The most obvious lacuna in interpreting these results is the overlaying of forest extent and deforestation information with property boundaries. Such information is needed for enforcement of restrictions on deforestation. It would also provide a key to a better understanding of the social dynamics of deforestation and other land-use changes. The most important decisions are made at the level of properties, not at levels of municípios, states or grid cells.
Models are needed that are capable of making forecasts and assessing the results of policy changes designed to maintain environmental functions of tropical forests and provide a sustainable livelihood to their human populations. Dale and Rauscher (1994) have reviewed the various types of models available for assessing the impacts of climate change on forests. Obtaining and interpreting information, while insufficient in itself, is currently one of the impediments to developing mechanisms for maintaining Amazonian forest that are based on the 'right' reasons: the environmental services it provides.
Some of the major causal relationships through which possible climatic changes could affect the forest's total biomass stock were presented earlier (Figure 6). Much remains to be done before our understanding of many of these relationships is sufficient to allow reliable predictions, but some conclusions are obvious. The number of ways in which climatic changes can provoke forest degradation and loss, processes which themselves reinforce the unfavorable climatic changes, indicates the importance of efforts to slow both deforestation and climatic change.
VI.) ACKNOWLEDGMENTS
The Pew Scholars Program in Conservation and the Environment provided funding. I thank S.V. Wilson and two anonymous reviewers for comments on the manuscript.
VII.) REFERENCES
Allegretti, M.H., 1990. Extractive reserves: An alternative for reconciling development and environmental conservation in Amazonia. In: A.B. Anderson (Editor), Alternatives to Deforestation: Steps toward Sustainable Use of Amazonian Rain Forest. Columbia University Press, New York, pp. 252-264.
Anderson, A.B., 1990. Smokestacks in the rainforest: Industrial development and deforestation in the Amazon Basin. World Development, 18: 1556-1570.
Bazzaz, F.A., 1990. The response of
natural ecosystems to the rising global CO2 levels. Annual Review of Ecological Systems, 21: 167-196.
Brazil, Presidência da República, Instituto Brasileiro de Geografia e
Estatística (IBGE), 1989. Anuário Estatístico do Brasil 1989. Vol. 49.
IBGE, Rio de Janeiro, 716 pp.
Brown, S. and Lugo, A.E., 1984. Biomass of tropical forests: A new estimate based on forest volumes. Science, 223: 1290-1293.
Brown, S. and Lugo, A.E., 1992a. Aboveground biomass estimates for tropical moist forests of the Brazilian Amazon. Interciencia, 17(1): 8-18.
Brown, S. and Lugo, A.E., 1992b. Biomass
estimates for Brazil's Amazonian moist forests. In: Forest '90: Anais do Primeiro Simpósio
Internacional de Estudos Ambientais em Florestas Tropicais Úmidas.
Biosfera--Sociedade Brasileira para a Valorização do Meio Ambiente, Rio de
Janeiro, pp. 46-52.
Brown, S. and Lugo, A.E., 1992c. Biomass of Brazilian Amazonian forests: The need for good science. Interciencia, 17(4): 201-203.
Brown, S., Gillespie, A.J.R. and Lugo,
A.E., 1989. Biomass estimation methods for tropical forests with applications
to forest inventory data. For.
Sci., 35(4):
881-902.
Buchmann, J., 1981. Um Estudo sobre a Influência de Fenômenos
Meteorológicos Extratropicais na Variação do Clima do Nordeste Brasileiro.
Comissão de Divulgação e Publicação da Coordenação dos Programas de
Pós-Graduação de Engenharia (COPPE), Universidade Federal do Rio de Janeiro
(UFRJ), Rio de Janeiro, 123 pp.
Cammell, M.E. and Knight, J.D., 1992.
Effects of climatic change on the population dynamics of crop pests. Adv. Ecolog. Res., 22: 117-162.
Castro, N.R., 1989. Perspectivas de desenvolvimento regional. In: Perspectivas
da Economia Brasileira. Instituto Nacional de Pesquisas Econônico-Sociais
(INPES)/Instituto de Pesquisa Econômica Aplicada (IPEA), Rio de Janeiro, pp.
287-318.
Condon, M.A., Sasek, T.W. and Strain, B.R., 1992. Allocation patterns in two tropical vines in response to increased atmospheric CO2. Functional Ecol., 6(6): 680-685.
Coutinho, L.M., 1982. Ecological effects of fire in Brazilian cerrado. In: B.J. Huntley and B.H. Walker (Editors), Ecology of Tropical Savannas. Springer, Berlin, Germany, pp. 273-291.
Coutinho, L.M., 1990. Fire in the ecology of the Brazilian Cerrado. In: J.G. Goldammer (Editor), Fire in the Tropical Biota: Ecosystem Processes and Global Challenges. Springer‑Verlag, Heidelberg, Germany, pp. 82-105.
Dale, V.H. and Rauscher, H.M., 1994.
Assessing impacts of climate change on forests: The state of biological modeling. Climatic Change 28(1-2): 65-90.
da Mota, F.S., 1981. Meteorologia Agrícola. Nobel, São Paulo,
Brazil. 376 pp.
Dantas, M., 1979. Pastagens da Amazonia Central: Ecologia e fauna de solo. Acta Amazonica, 9(2): suplemento: 1‑54.
Eagleson, P.S., 1986. The emergence of global‑scale hydrology. Water Resources Res., 22(9): 6s‑14s.
FAO (Food and Agriculture Organization of the United Nations), 1993. Forestry Statistics Today for Tomorrow: 1961-1991...2010. FAO, Rome, 45 pp.
FAO (Food and Agriculture Organization of the United Nations), 1994. Forest Resources Assessment 1990: Tropical Countries. FAO Forestry Paper 112. FAO, Rome.
Fearnside, P.M., 1979. O processo de desertificação e os riscos de sua ocorrencia no Brasil. Acta Amazonica, 9(2): 393‑400.
Fearnside, P.M., 1984. Simulation of meteorological parameters for estimating human carrying capacity in Brazil's Transamazon Highway colonization area. Trop. Ecol., 25(1): 134‑142.
Fearnside, P.M., 1985a. Environmental Change and Deforestation in the Brazilian Amazon. In: J. Hemming (Editor), Change in the Amazon Basin: Man's Impact on Forests and Rivers. Manchester University Press, Manchester, U.K., pp. 70‑89.
Fearnside, P.M., 1985b. Brazil's Amazon forest and the global carbon problem. Interciencia, 10(4): 179‑186.
Fearnside, P.M., 1986a. Human Carrying Capacity of the Brazilian Rainforest. Columbia University Press, New York, 293 pp.
Fearnside, P.M., 1986b. Spatial concentration of deforestation in the Brazilian Amazon. Ambio, 15(2): 72‑79.
Fearnside, P.M., 1986c. Brazil's Amazon forest and the global carbon problem: Reply to Lugo and Brown. Interciencia, 11(2): 58-64.
Fearnside, P.M., 1987a. Causes of deforestation in the Brazilian Amazon. In: R.F Dickinson (Editor), The Geophysiology of Amazonia: Vegetation and Climate Interactions. John Wiley & Sons, New York, pp. 37‑61.
Fearnside, P.M., 1987b. Deforestation and international economic development projects in Brazilian Amazonia. Cons. Biol., 1(3): 214‑221.
Fearnside, P.M., 1988. Jari at age 19:
Lessons for Brazil's silvicultural plans at Carajás. Interciencia, 13(1): 12‑24; 13(2): 95.
Fearnside, P.M., 1989a. A Ocupação Humana de Rondônia: Impactos,
Limites e Planejamento. CNPq Relatórios de Pesquisa No. 5. Conselho
Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Brasilia, 76 pp.
Fearnside, P.M., 1989b. Extractive reserves in Brazilian Amazonia: Opportunity to maintain tropical rain forest under sustainable use. BioScience, 39(6): 387‑393.
Fearnside, P.M., 1989c. Forest management in Amazonia: The need for new criteria in evaluating development options. For. Ecol. Manage., 27: 61‑79.
Fearnside, P.M., 1989d. The charcoal of Carajás: Pig‑iron smelting threatens the forests of Brazil's Eastern Amazon Region. Ambio, 18: 141‑143.
Fearnside, P.M., 1990a. Predominant land uses in the Brazilian Amazon. In: A.B. Anderson (Editor), Alternatives to Deforestation: Towards Sustainable Use of the Amazon Rain Forest. Columbia University Press, New York, pp. 235-251.
Fearnside, P.M. 1990b. The rate and extent of deforestation in Brazilian Amazonia. Environmental Conserv., 17(3): 213-226.
Fearnside, P.M., 1992a. Greenhouse Gas Emissions from Deforestation in the Brazilian Amazon. Carbon Emissions and Sequestration in Forests: Case Studies from Developing Countries. Volume 2. LBL-32758, UC-402. Climate Change Division, U.S. Environmental Protection Agency, Washington, DC, and Energy and Environment Division, Lawrence Berkeley Laboratory (LBL), University of California (UC), Berkeley, California, 73 pp.
Fearnside, P.M., 1992b. Forest biomass in
Brazilian Amazonia: comments on the estimate by Brown and Lugo. Interciencia, 17(1): 19-27.
Fearnside, P.M., 1993a. Desmatamento na Amazônia: Quem tem razão‑‑o INPE ou a NASA? Ciência Hoje, 16(96): 6-8.
Fearnside, P.M., 1993b. Deforestation in Brazilian Amazonia: The effect of population and land tenure. Ambio, 22(8): 537-545.
Fearnside, P.M., 1993c. Biomass of Brazil's Amazonian forests: Reply to Brown and Lugo revisited. Interciencia, 18(1): 5-7.
Fearnside, P.M., 1995a. Amazonian deforestation and global warming: Carbon stocks in vegetation replacing Brazil's Amazon forest. For. Ecol. Manage. (forthcoming).
Fearnside, P.M., 1995b. Biomass of
Brazil's Amazonian forests. Unpublished
manuscript, Department of Ecology, Instituto Nacional de Pesquisas da Amazônia
(INPA), Manaus, Amazonas, Brazil.
Fearnside, P.M. and Rankin, J.M., 1980. Jari and development in the Brazilian Amazon. Interciencia, 5(3): 146‑156.
Fearnside, P.M. and Rankin, J.M., 1985. Jari revisited: Changes and the outlook for sustainability in Amazonia's largest silvicultural estate. Interciencia, 10(3): 121‑129.
Fearnside, P.M., Leal Filho, N. and Fernandes, P.M., 1993. Rainforest burning and the global carbon budget: Biomass, combustion efficiency and charcoal formation in the Brazilian Amazon. J. Geophys. Res., 98(D9): 16,733-16,743.
Fearnside, P.M., Tardin, A.T. and Meira
Filho, L.G., 1990. Deforestation rate in Brazilian Amazonia. Instituto de Pesquisas Espaciais
(INPE), São José dos Campos, São Paulo (Preprint), 8 pp.
Gates, W.L., Mitchell, J.F.B., Boer, G.J., Cubasch, U. and Meleshko, V.P., 1992. Climate modelling, climate prediction and model validation. In: J.T. Houghton, B.A. Callander and S.K. Varney (Editors), Climate Change 1992: The Supplementary Report to the IPCC Scientific Assessment. Cambridge University Press, Cambridge, U.K., pp. 97-134.
Grace, J., Lloyd, J., McIntyre, J., Miranda, A., Meir, P., Miranda, H., Nobre, C., Moncrieff, J., Massheder, J., Yadvinder, M., Wright, I. and Gash, J., 1995. Fluxes of carbon dioxide and water vapour over an undisturbed tropical rain forest in South-West Amazonia. Global Change Biology 1: 1-12.
Grainger, A., 1990. Modelling the impact of alternative afforestation strategies to reduce carbon dioxide emissions. In: Intergovernmental Panel on Climate Change (IPCC), Response Strategies Working Group (RSWG), Subgroup on Agriculture, Forestry and other Human Activities (AFOS), Proceedings of the Conference on Tropical Forestry Response Options to Global Climate Change. U.S. Environmental Protection Agency, Office of Policy Assessment (USEPA-OPA, PM221), Washington, DC, pp. 95-104.
Hecht, S.B., 1993. The logic of livestock and deforestation in Amazonia. BioScience, 43(10): 687-695.
Hecht, S.B., Norgaard, R.B. and Possio, C., 1988. The economics of cattle ranching in eastern Amazonia. Interciencia, 13(5): 233-240.
Janzen, D.H., 1967. Why mountain passes are higher in the tropics. Am. Naturalist, 101: 233-249.
Janzen, D.H., 1970. The unexploited tropics. Ecolog. Soc. Amer. Bull., 51: 4‑7.
Janzen, D.H., 1973. Tropical agroecosystems: habitats misunderstood by the temperate zones, mismanaged by the tropics. Science, 182: 1212‑1219.
Kapos, V., 1989. Effects of isolation on the water status of forest patches in the Brazilian Amazon. J. Trop. Ecol., 5: 173-185.
Kapos, V., Ganade, G., Matusi, E. and Victoria, R.L., 1993. Delta 13C as an indicator of edge effects in tropical rainforest reserves. J. Ecol., 81: 425-432.
Leopoldo, P.R., Franken, W. and Salati, E., 1982. Balanço hídrico de pequena bacia hidrográfica em floresta amazônica de terra firme. Acta Amazonica, 12(2): 333‑337.
Liss, P.S. and Crane, A.J., 1983. Man-made Carbon Dioxide and Climate Change: A Review of Scientific Problems. GEO Books, Norwich, CT, 127 pp.
Lovejoy, T.E., Rankin, J.M., Bierregaard, R.O., Jr., Brown, K.S., Jr., Emmons, L.H. and Van der Voort, M.E., 1984. Ecosystem decay of Amazon forest remnants. In: M.H. Nitecki (Editor), Extinctions. University of Chicago Press, Chicago, IL, pp. 295‑325.
Lugo, A.E. and Brown, S., 1986. Brazil's Amazon forest and the global carbon problem. Interciencia, 11(2): 57-58.
Lugo, A.E. and Brown, S., 1992. Tropical forests as sinks of atmospheric carbon. For. Ecol. Manage., 54: 239-255.
Malingreau, J.P., Stephens, G. and
Fellows, L., 1985. Remote sensing of forest fires: Kalimantan and North Borneo
in 1982‑83. Ambio, 14(6): 314‑321.
Marques, J., dos Santos, J.M., Villa Nova, N.A. and Salati, E., 1977. Precipitable water and water vapor flux between Belém and Manaus. Acta Amazonica, 7(3): 355‑362.
McWilliam, A.-L.C., Roberts, J.M., Cabral, O.M.R., Leitão, M.V.B.R., de Costa, A.C.L., Maitelli, G.T. and Zamparoni, C.A.G.P., 1993. Leaf-area index and above-ground biomass of terra firme rain forest and adjacent clearings in Amazonia. Functional Ecol., 7(3): 310-317.
Meggers, B.J., 1994. Archeological evidence for the impact of mega-Niño events on Amazonia during the past two millenia. Climatic Change, 28(1-2): 321-338.
Molion, L.C.B., 1975. A Climatonomic Study of the Energy and Moisture Fluxes of the Amazonas Basin with Considerations of Deforestation Effects. Ph.D. Diss. Climatology, University of Wisconsin, Madison. University Microfilms International, Ann Arbor, MI.
Myers, N., 1993. Environmental refugees
in a globally warmed world. BioScience, 43(11): 752-761.
Nepstad, D., de Carvalho, C.R., Davidson, E., Jipp, P., Lefebvre, P., Negreiros, G.H., da Silva, E.D., Stone, T., Trumbore, S. and Vieira, S., 1994. The role of deep roots in the hydrological and carbon cycles of Amazonian forests and pastures. Nature, 372: 666-669.
Parry, M., 1992. The potential effect of climate changes on agriculture and land use. Adv. Ecolog. Res., 22: 63-91.
Phillips, O. and Gentry, A.H., 1994. Increasing turnover through time in tropical forests. Science, 263: 954-958.
Rankin-de-Merona, J.M., Hutchings, R.W. and Lovejoy, T.E., 1990. Tree mortality and recruitment over a five-year period in undisturbed upland rainforest of the Central Amazon. In: A.H. Gentry (Editor), Four Neotropical Rainforests. Yale University Press, New Haven, CT, pp. 573-584.
Reis, E.J. and Margulis, S., 1991. Perspectivas Econômicas do
Desflorestamento da Amazônia. Textos para Discussão No. 215. Instituto de Pesquisa Econômica
Aplicada (IPEA), Brasilia, 47 pp.
Revelle, R., 1982. Carbon dioxide and world climate. Sci. Am., 247(2): 33‑41.
Richey, J.E., Mertes, L.A.K., Dunne, T., Victória, R.L., Forsberg, B.R., Tancredi, A.C.N.S. and Oliveira, E., 1989. Sources and routing of the Amazon River floodwave. Global Biogeochem. Cycles, 3: 191-204.
Salati, E. and Vose, P.B., 1984. Amazon
Basin: A system in equilibrium. Science, 225: 129‑138.
Salati, E., Marques, J. and Molion, L.C.B., 1978. Origem e distribuição das chuvas na Amazônia. Interciencia, 3(4): 200‑206.
Salati, E., Dall'Olio, A., Matusi, E. and Gat, J.R., 1979. Recycling of water in the Brazilian Amazon Basin: An isotopic study. Water Resources Res., 15: 1250‑1258.
Schubart, H.O.R., Junk, W.J. and Petrere,
M., Jr., 1976. Sumário de
ecologia Amazônica. Ciência e Cultura, 28(5): 507‑509.
Setzer, A.W. and Pereira, M.C., 1991. Amazonia biomass burning in 1987 and an estimate of their tropospheric emissions. Ambio, 20(1): 19-22.
Shukla, J., Nobre, C. and Sellers, P., 1990. Amazon deforestation and climate change. Science, 247: 1322-1325.
Skole, D. and Tucker, C., 1993. Tropical deforestation and habitat fragmentation in the Amazon: Satellite data from 1978 to 1988. Science, 260: 1905-1910.
Solomon, A.M., Prentice, I.C., Leemann, R. and Cramer, W.P., 1993. The interaction of climate and land use in future terrestrial carbon storage and release. Water, Air and Soil Pollut., 70: 595-614.
Swap, R., Garstang, M. and Greco, S., 1992. Saharan dust in the Amazon Basin. Tellus, 44B: 133-149.
Talbot, R.W., Andreae, M.O., Berresheim, H., Artaxo, P., Garstang, M., Harriss, R.C., Beecher, K.M. and Li, S.M., 1990. Aerosol chemistry during the wet season in Central Amazonia: The influence of long-range transport. J. Geophys. Res., 95: 16,955-16,969.
Uhl, C. and Buschbacher, R., 1985. A disturbing synergism between cattle‑ranch burning practices and selective tree harvesting in the eastern Amazon. Biotropica, 17(4): 265‑268.
Uhl, C. and Kauffman, J.B., 1990. Deforestation, fire susceptibility, and potential tree responses to fire in the Eastern Amazon. Ecology, 71(2): 437-449.
Uhl, C. and Vieira, I.C.G., 1989.
Ecological impacts of selective logging in the Brazilian Amazon: A case study
from the Paragominas region in the state of Pará. Biotropica, 21: 98-106.
Veríssimo, A., Barreto, P., Tarifa, R. and Uhl, C., 1995. Extraction
of a high-value natural resource in Amazonia: The case of mahogany. For. Ecol. Manage., 72: 39-60.
Villa Nova, N.A., Salati, E. and Matusi, E., 1976. Estimativa da
evapotranspiração na Bacia Amazônica. Acta Amazonica, 6(2): 215‑228.
Watson, R.T., Meira Filho, L.G., Sanhueza, E. and Janetos, A., 1992. Greenhouse gases: Sources and sinks. In: J.T. Houghton, B.A. Callander and S.K. Varney (Editors), Climate Change 1992: The Supplementary Report to the IPCC Scientific Assessment. Cambridge University Press, Cambridge, pp. 25-46.
Watson, R.T., Rodhe, H., Oeschger, H. and Siegenthaler, U., 1990. Greenhouse gases and aerosols. In: J.T. Houghton, G.J. Jenkins and J.J. Ephraums (Editors), Climate Change: The IPCC Scientific Assessment. Cambridge University Press, Cambridge, pp. 1-40.
Zuidema, G., Van den Born, G.J., Alcamo, J. and Kreileman, G.J.J. 1994. Simulating changes in global land cover as affected by economic and climatic factors. Water, Air and Soil Pollut., 76(1-2): 163-198.
FIGURE CAPTIONS
Figure 1 --Brazil and the Legal Amazon region.
Figure 6 --Causal loop diagram of links of possible climatic changes to forest biomass stock. The signs represent the direction of change of the quantity at the head of the arrow given an increase in the quantity at the tail of the arrow.
Figure 7 --Industrial roundwood export and consumption from domestic sources.
Figure 8 --Domestic consumption of sawlogs.
Figure 9 --Domestic consumption of pulp and firewood (including charcoal and charcoal-derived pig iron)
Figure 10 --A) Logging intensity under different climatic change scenarios.
B) Area logged per year under different climatic change scenarios.
Fig. 1
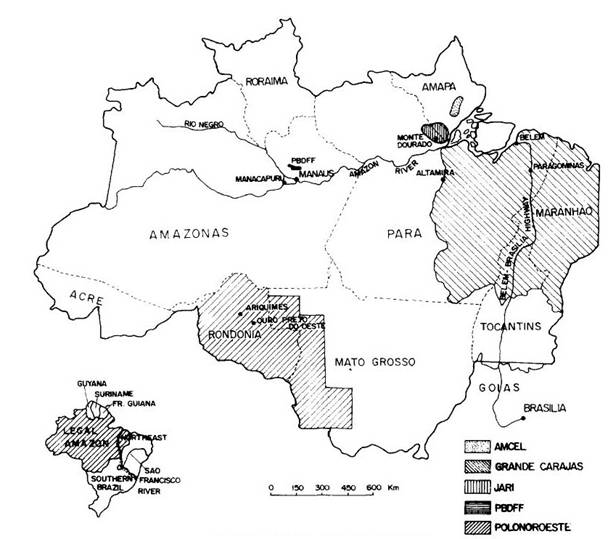
Fig. 2
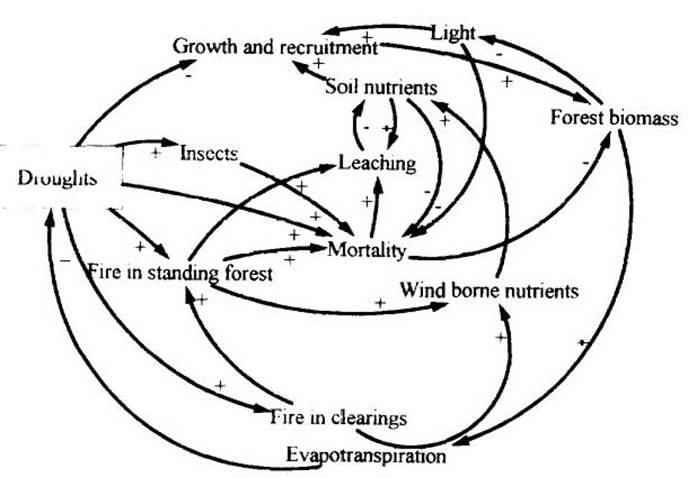
Fig. 3
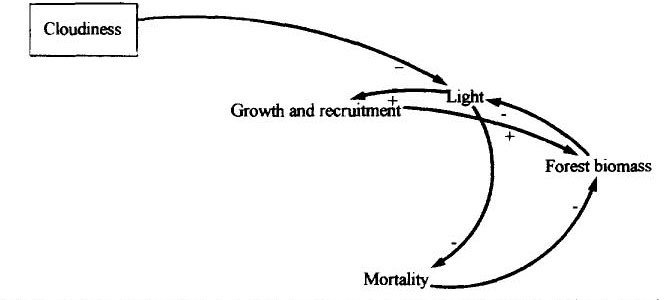
Fig. 4
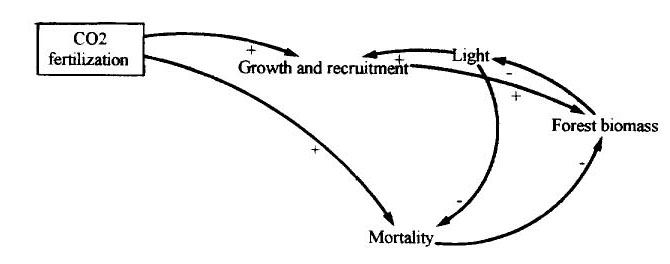
Fig. 5
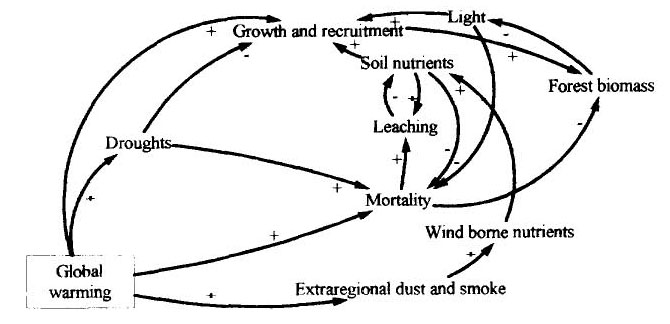
Fig. 6

Fig. 7
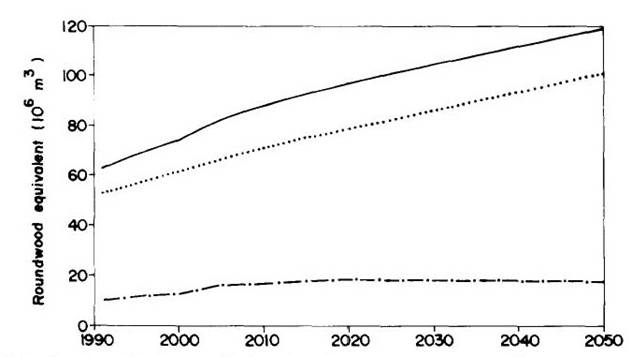
Fig. 8
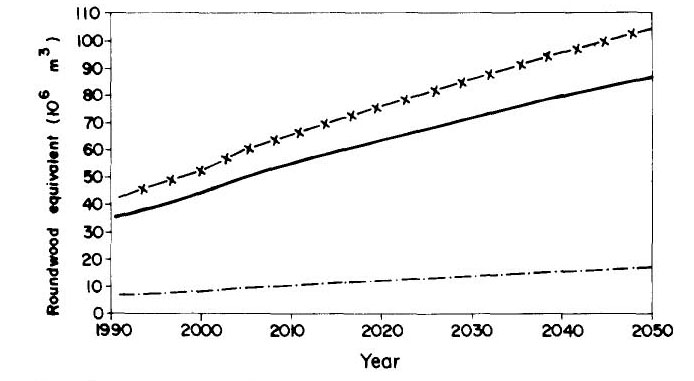
Fig. 9
Fig. 10