Fig. 1
Fig. 2
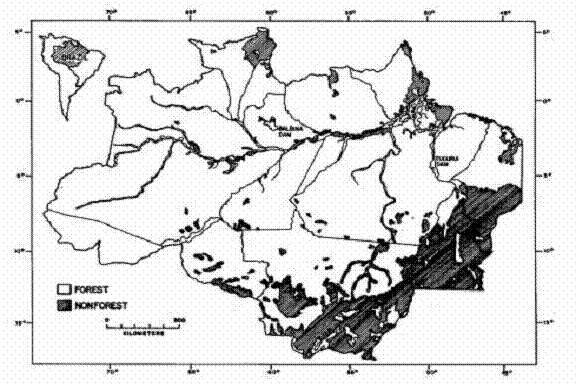
Fig. 3

Fig. 4
The
text that follows is a PREPRINT.
Please cite as:
Fearnside, P.M. 1997. Greenhouse gases from deforestation in
Brazilian Amazonia: Net committed emissions. Climatic Change 35(3):
321-360.
ISSN: 0165-0009
Copyright: Springer.
The original publication is available
at www.springerlink.com
GREENHOUSE GASES
FROM DEFORESTATION IN BRAZILIAN AMAZONIA:
NET COMMITTED EMISSIONS
Philip
M. Fearnside
Department of Ecology
National
Institute for Research
in the Amazon (INPA)
C.P. 478
69011-970 Manaus-Amazonas
BRAZIL
Fax: 55-92-236-3822
Submitted to Climatic Change
22
Sept. 1995
revised
19 Feb. 1996
23
Feb. 1996
9
Oct. 1996
CONTENTS
List of Tables
............................................... ii
List of Figures
.............................................. iv
Abstract
..................................................... v
I.)
INTRODUCTION ........................................... 1
II.)
EXTENT AND RATE OF DEFORESTATION ....................... 5
III.)
BIOMASS OF AMAZONIAN FORESTS ........................... 6
IV.)
TRANSFORMATIONS OF GROSS CARBON STOCKS
A.)
Land Uses Replacing the Forest ................. 12
B.)
Fate of Biomass Carbon Stocks .................. 14
V.)
SOURCES AND SINKS OF GREENHOUSE GASES
A.)
Burning ........................................ 16
B.)
Removal of Intact Forest Sources and Sinks ..... 19
C.)
Soil Carbon .................................... 20
D.)
Termites and Decay ............................. 24
E.)
Cattle and Pasture ............................. 26
F.)
Hydroelectric Dams ............................. 28
VI.)
NET COMMITTED EMISSIONS ............................... 31
VII.)
NOTE .................................................. 33
VIII.) ACKNOWLEDGMENTS
....................................... 33
IX.)
REFERENCES ............................................ 35
Figure Legends
Tables
Figures
LIST OF TABLES
Table
I.Extent of deforestation in the Brazilian Legal Amazon.
Table
II.Rate of deforestation in the Brazilian Legal Amazon.
Table
III. Vegetation types in the Brazilian Legal Amazon.
Table
IV.Area of natural vegetation present in the Brazilian Legal Amazon.
Table
V.Biomass per hectare: means by ecoregion, vegetation type and state.
Table
VI.Approximate biomass cleared in 1990 in each ecoregion in the Brazilian Legal
Amazon.
Table
VII. Markov matrix for land use
transformations after deforestation.
Table
VIII.Replacement vegetation weighted biomass calculation.
Table
IX.Parameters for transformations of gross carbon stocks.
Table
X.Parameters for carbon emissions.
Table
XI.Parameters for other sources of greenhouse gases from land-use change.
Table
XII. Trace gas parameters.
Table
XIII.Soil carbon parameters and calculations.
Table
XIV.Low trace gas scenario: Net committed emissions by source for 1990 clearing
in the Legal Amazon.
Table
XV.High trace gas scenario: Net committed emissions by source for 1990 clearing
in the Legal Amazon.
Table
XVI.Net committed emissions from 1990 deforestation with CO2-equivalent
carbon, 100-year time horizon.
LIST OF FIGURES
Fig.
1.Brazil's Legal Amazon region, with locations mentioned in the text.
Fig.
2.Forest and nonforest in the Brazilian Legal Amazon (Source: Fearnside and
Ferraz, 1995).
Fig.
3.Carbon transformations through a typical 10-year sequence of clearing,
burning and reburning (updated from: Fearnside, 1991).
Fig.
4.Partitioning of carbon between type of release and emitted gas (Low trace gas
scenario) (updated from: Fearnside, 1991).
Abstract.
Deforestation in Brazilian Amazonia is a significant source of
greenhouse gases today and, with almost 90% of the originally forested area
still uncleared, is a very large potential source of future emissions. The 1990 rate of loss of forest (13.8 X 103
km2/year) and cerrado savanna (approximately 5 X 103
km2/year) was responsible for releasing approximately 261 X 106
metric tons of carbon (106 t C) in the form of CO2, or
274-285 X 106 t of CO2-equivalent C considering IPCC 1994
global warming potentials for trace gases over a 100-year horizon. These calculations consider conversion to a landscape
of agriculture, productive pasture, degraded pasture, secondary forest, and
regenerated forest in the proportions corresponding to the equilibrium
condition implied by current land-use patterns.
Emissions are expressed as "net committed emissions," or the
gases released over a period of years as the carbon stock in each hectare
deforested approaches a new equilibrium in the landscape that replaces the
original forest. For low and high trace
gas scenarios, respectively, 1990 clearing produced net committed emissions (in
106 t of gas) of 957-958 for CO2, 1.10-1.42 for CH4,
28-35 for CO, 0.06-0.16 for N2O, 0.74-0.74 for NOx and
0.58-1.16 for non-methane hydrocarbons.
1. INTRODUCTION
The present paper offers a structure for
analyzing the greenhouse gas contribution of deforestation in Brazilian
Amazonia (Figure 1). It is hoped that
this structure will prove valuable beyond the short time that the series of
numbers for greenhouse gas emissions presented here remains the current best
estimate. As the rates and locations of
deforestation activity change, and as better data become available on these and
other important factors, the estimates can be continually updated. Deforestation rates declined over the period
from 1987 to 1991, but this is largely explained by Brazil's deepening economic
recession, and cannot be extrapolated into the future (Fearnside, 1993a).
(Figure 1 here)
A variety of measures exists for expressing
greenhouse gas emissions from tropical deforestation (see Makundi et al.,
1992). One of these is net committed
emissions, which considers the emissions and uptake that will occur as the
landscape approaches a new equilibrium condition in a given deforested area
(here the 13.8 X 103 km2 of Brazil's Amazonian forest
that was cut in 1990). The "prompt
emissions" (emissions entering the atmosphere in the year of clearing) are
considered along with the "delayed emissions" (emissions that will
enter the atmosphere in future years), as well as the corresponding uptake as
replacement vegetation regrows on the deforested sites. Not included are trace gas emissions from the
burning and decomposition of secondary forest and pasture biomass in the
replacement landscape, although both trace gas and carbon dioxide fluxes are
included for emissions originating from remains of the original forest biomass,
from loss of intact forest sources and sinks, and from soil carbon pools. Net committed emissions are calculated as the
difference between the carbon stocks in the forest and the equilibrium
replacement landscape, with trace gas fluxes estimated based on fractions of
the biomass that burn or decompose following different pathways.
Net committed emissions are not the same as
the annual balance of emissions from the region. An annual balance calculation would consider
the entire region (not just the part deforested in a single year), and would
consider the fluxes of gases entering and leaving the region both through
prompt emissions in the newly deforested areas and through the
"inherited" emissions and uptakes in the clearings of different ages
throughout the landscape. Inherited
emissions and uptakes are the fluxes occurring in the year in question that are
the result of clearing activity in previous years, for example, from
decomposition or reburning of the remaining biomass of the original
forest. The annual balance also includes
trace gases from secondary forest and pasture burning and decomposition. The Framework Convention on Climate Change,
signed in Rio de Janeiro in June 1992 by 155 countries plus the European Union,
requires national estimates of fluxes and stocks for use in calculating the
annual balance of emissions for each country.
Presumably, the annual balance will form the basis for assigning
responsibility for global warming in any protocols that may be negotiated under
the Framework Convention.
Net committed emissions has several
advantages over the annual balance as a measure of the impact of tropical
deforestation, for example, for purposes of comparing the impacts of
deforestation to those of fossil fuel combustion. Burning fossil fuels releases all gases in a
single pulse, whereas deforestation sets in motion a process that commits
emissions for a number of years after the trees are cut. Comparisons limited to prompt emissions from
the two sources therefore grossly understate the impact of deforestation. The inherited emissions included in the
annual balance avoids much of this distortion, but changes in the deforestation
rate alter its appropriateness as a measure of the importance of
deforestation. When clearing rates are
increasing, the annual balance will understate the impact of deforestation, and
when clearing rates are decreasing, it will overstate it. Except for the relatively small contribution
of trace gases from secondary forest and pasture biomass, the annual balance
and net committed emissions would be equal if deforestation rates were constant
over an extended period of years. Using
net committed emissions as a measure of the importance of deforestation is a
more appropriate measure than the annual balance for assessment of
deforestation policy questions in terms of the global interest (as
opposed to each country's national interest under the Framework Convention).
Although better than the annual balance,
net committed emissions is not ideal as the measure of deforestation's
impact. The time when emissions occur is
important, as human societies place a higher value on avoiding short-term
impacts than on impacts far in the future.
Although this preference is reflected in the time horizon of the global
warming potentials used to express different trace gases in terms of carbon
dioxide equivalents, the computations only include time preference with respect
to the atmospheric load originating from a single pulse of gas, not the timing
of the fluxes themselves in the case of delayed emissions. To the extent that deforestation has remained
constant, net committed emissions are the same as the annual balance and
knowledge of the timing of releases becomes unnecessary, thus greatly
simplifying both data requirements and computation. A better index than net committed emissions
would be provided by what may be called "time preference weighted
emissions"--a measure derived from a non-equilibrium calculation using
explicit fluxes for each year in the cleared area, and weighting these using
both a time horizon and a scheme for assigning time preference (as by
discounting or other alternative procedures).
Discount rates or other time preference adjustments appropriate for such
calculations are unlikely to be the same as those employed in financial
analyses (Fearnside, 1995a).
2. EXTENT AND RATE OF
DEFORESTATION
The present paper uses estimates of the
extent and rate of deforestation by state derived from LANDSAT imagery (Tables
I and II). The cumulative area
deforested through 1991 had reached 427 X 103 km2
(including old clearings and hydroelectric dams), or 10.7% of the 4 X 106
km2 originally forested portion of Brazil's 5 X 106 km2
Legal Amazon region. Forest loss from
1978 through 1988 proceeded at an average of 20.4 X 103 km2/year
(Fearnside, 1993b); this rate estimate is derived, with a variety of
adjustments, from estimates of deforestation extent in 1978 (Skole and Tucker,
1993) and in 1988 (Fearnside et al., 1990, see Fearnside, 1993a). Deforestation rate fell to 19.2 X 103
km2/year in 1989, 13.8 X 103 km2/year in 1990,
and 11.1 X 103 km2/year in 1991 (Brazil, INPE, 1992, see
Fearnside, 1993a). The 1991 rate of
forest loss was 20% less than the 1990 rate on which the emissions calculations
in this paper are based.
(Tables I and II here)
The above rates cover only loss of primary
forest within the portion of the region that was originally forested. Rates of conversion of the nonforest area,
mainly cerrado (central Brazilian dry scrub savanna) are far less
certain, but fortunately have less impact on greenhouse gas calculations due to
the much lower biomass of savanna vegetation.
The cerrado clearing rate for 1990 is assumed to be 5 X 103
km2/year, a value lower than the 10 X 103 km2/year
used previously for 1990 (Fearnside, 1992a), and much lower than the 18 X 103
km2/year estimated for 1988 (Fearnside, 1990). Based on comparison of Brazil's 1970 and 1985
agricultural censuses, Klink et al. (1994) estimated that 20 X 103
km2/year of cerrado were cleared over that period, including
areas outside of the Legal Amazon. The cerrado
loss rate used in my present emissions estimate is believed to be conservative.
The rate of deforestation, together with
the biomass of forest being cleared, affects the current (as opposed to
potential) contribution of deforestation to the greenhouse effect. The rate of clearing has been calculated for
each state (Table II), and is apportioned among various forest types within
each state by assuming that, within each state, each forest type is cleared in
proportion to the area in which it occurs outside of protected areas.
3. BIOMASS OF
AMAZONIAN FORESTS
The initial biomass of the vegetation is an
important factor affecting the magnitude of greenhouse gas emissions from
deforestation. The biomass estimate used
in the present paper-- 434 metric tons per hectare (t/ha) pre-logging total
biomass for forests cleared in 1990 [post-logging biomass is 407 t/ha]--is
based on much more data than earlier estimates and is derived as a weighted
average from estimates that are disaggregated by state and forest type. The estimate indicates a substantial increase
in biomass per hectare estimated for locations currently the focus of
deforestation activity in Amazonia. It
more than doubles the 155.1 t/ha value for total biomass derived by Brown and
Lugo (1984) from FAO forest volume surveys for "tropical American
undisturbed productive broadleafed forests" that has been used in recent
global carbon balance calculations (e.g., Detwiler and Hall, 1988). It is also much higher than the 169.8 t/ha
above-ground estimate by Brown et al. (1989) used as total biomass by
Houghton (1991) for carbon emission estimates.
Hall and Uhlig (1991) used the volume and expansion factor from the
Brown et al. (1989) study, plus a correction for below-ground biomass,
to obtain an estimate of 98.2 t C/ha (196.4 t biomass/ha) for undisturbed
forests in "Brazil." The
estimate used in the present study is also higher than the 211 t/ha total
biomass estimated for areas cleared in 1988 for emissions calculations
(Fearnside, 1991); a major reason for the increase is better data (derived from
forest inventories of Brazil, Projeto RADAMBRASIL, 1973-1983) for biomass in
the southern portion of the region where deforestation activity is
concentrated. The estimate also has
improved information on wood density and on below-ground biomass.
The different types of vegetation present
in the Legal Amazon have been measured (Fearnside and Ferraz, 1995) from a
digitized version of the 1:5,000,000 scale vegetation map of Brazil published
by the Brazilian Institute for Geography and Statistics (IBGE) and the
Brazilian Institute for Forestry Development (IBDF--since incorporated into the
Brazilian Institute for the Environment and Renewable Natural Resources -
IBAMA) (Brazil, IBGE and IBDF, 1988).
The IBGE/IBDF (IBAMA) map code used indicates 28 vegetation types within
the Brazilian Legal Amazon, of which 19 are considered here to be forest (Table
III). This is a liberal definition of
forest, including all ecotones between a forest and a nonforest vegetation type
such as cerrado. So defined, the
area of forest present according to the map totals 3.7 X 106 km2,
or 74% of the 5 X 106 km2 Legal Amazon. The area originally forested totals 4.3 X 106
km2 (Table IV). The areas
that were originally forest and nonforest using this definition are mapped in
Figure 2. The deforestation estimate
used here has as its base a definition of originally forested area
corresponding to approximately 4.0 X 106 km2, therefore
implying some remaining inconsistency.(*)
(Tables III and IV and Figure 2 here)
Because the Legal Amazon is so big, each of
its nine states being the size of countries in many parts of the world,
vegetation with the same map code in different states cannot be assumed to have
the same biomass. Considering each
vegetation type in each state as a separate unit, here designated "ecoregions,"
there are a total of 111 different ecoregions in the Legal Amazon, of which 78
are "forest."
In order to estimate the area of each
forest type being cleared annually in 1990, it was assumed that forests within
each state are cleared in proportion to the area of each type (outside of parks
and other legally protected areas).
Although protected areas are not immune to deforestation, the small
amount of clearing activity currently taking place inside these areas is
undoubtedly insignificant from the standpoint of greenhouse gas emissions.
All biomass values given here and elsewhere
in this paper refer to oven dry weight biomass.
Unless otherwise noted, values are for total biomass, including both
above- and below-ground portions, and including dead vegetation (but not soil
carbon). All biomass fractions are
included (leaves, small trees, vines, understory, etc.). Values are expressed in terms of biomass,
rather than carbon (carbon content of biomass is 50%).
Biomass loading (biomass per hectare) of
the different forested ecoregions is estimated from forest volume inventories
in surveys carried out by the RADAMBRASIL project in the 1970s (Brazil, Projeto
RADAMBRASIL, 1973-1983) and by the Food and Agriculture Organization of the
United Nations (FAO) in the 1950s (Glerum, 1960; Heinsdijk, 1957,
1958a,b,c). A total of 2954 ha of usable
data has been extracted from these studies for vegetation types classified as
forest. Almost all of the FAO and
RADAMBRASIL data are from one-hectare sample plots.
The parameters used for deriving the
biomass estimates from the forest volume data lead to estimated biomass values
substantially higher than those derived by Brown and Lugo (1992) from the FAO
data and a summary of a portion of the RADAMBRASIL dataset. The difference is largely because of biomass
components omitted from the Brown and Lugo estimates, including palms, vines,
trees smaller than 10-cm DBH, dead biomass and below-ground biomass (Fearnside,
1992b, 1993c). Adjustments for these
components are made based on available direct measurements (Fearnside, nd-a).
The total biomass is derived for each
sample and the average for each ecoregion is calculated. Of the 78 forested ecoregions appearing on
the IBAMA (Brazil, IBGE and IBDF, 1988) map, 44 (56%) have forest volume data
available in the RADAMBRASIL or FAO datasets, and 34 (44%) do not. Fortunately, most of the ecoregions without
data are of relatively minor importance from the standpoint of current greenhouse
gas emissions. Of estimated biomass
cleared in 1990, they total only 21.5%.
For the ecoregions with no forest volume measurements, the mean biomass
for the areas sampled in the same forest type (in the other states) is used as
a substitute. For 5 of the 19 forest
types, no measurement exists for any state.
Forest types with no sample in any state represent only 1.0% of the
estimated biomass cleared in 1990.
Mean biomass per hectare in each of the 78
forest types, including the values substituted as described above, are
presented in Table V. It is evident that
significant variation exists among states and among forest types, with
pre-logging biomass loading ranging from 336 to 613 t/ha.
(Table V here)
The biomass stock in each ecoregion can be
calculated by multiplying the per-hectare biomass (Table V) by the area in
hectares (values from Table IV multiplied by 100 ha/km2). Table VI gives the approximate biomass stock
cleared in million t for each ecoregion in the Legal Amazon. For the region's forests as a whole, the mean
biomass loading (t/ha) for pre-logging biomass (weighted by the area of each
ecoregion present) is estimated at 464 t/ha (Table V). In Table VI the loading for pre-logging
biomass of forests cleared in 1990 (weighted by the deforestation rate in each
state) is calculated as 434 t/ha. The
pre-logging biomass in the areas cleared in 1990 is 6.5% lower than the average
in the region as a whole, a difference equivalent to 892 km2 of
forest clearing.
(Table VI here)
4. TRANSFORMATIONS OF
GROSS CARBON STOCKS
4.1 Land Uses
Replacing the Forest
Estimates of the impact of deforestation
have usually assumed that all deforested land is converted to cattle pasture
(the dominant land use in deforested areas in Brazilian Amazonia). Some have even assumed that the forest is
replaced with bare ground. Pasture has
been assumed to remain indefinitely as the replacement for forest in estimates
of net greenhouse gas emissions (e.g., Fearnside, 1985, 1987), and in
simulations of impact on the water cycle (e.g., Shukla et al., 1990) and
of the less-threatening changes in surface albedo (Dickinson and
Henderson-Sellers, 1988). The results of
such calculations are useful in identifying potential consequences of continued
deforestation, but are unrealistic as quantitative predictions of contributions
to climatic changes because the landscape that really replaces forest is not
only pasture. The principal reason for
using cattle pasture as the replacement vegetation has been the lack of more
realistic scenarios of the evolution of the landscape after its initial
conversion from forest to pasture. Here
a first approximation is made using a simple first-order Markov model of
transition probabilities between land-use classes (Fearnside, in press-a).
Markov matrices carry the assumption that
the transfer probabilities remain unaltered over time--something for which
there is no guarantee in practice.
However, in most agricultural systems the tendency has been for
population pressure to increase, leading to increased use intensity over time
and shorter periods in secondary forest, with resulting lower average biomass
for the landscape (e.g., Vermeer, 1970; UNESCO/UNEP/FAO, 1978). The assumption of constant transfer
probabilities therefore is conservative from the point of view of greenhouse
gas emissions. The assumption of
constant transition probabilities is also optimistic because degradation of
soil under pasture, combined with rainfall changes expected if the scale of
deforestation should greatly expand, are likely to make low-biomass
dysclimaxes, including grassy formations, the dominant land cover in a
deforested Amazonia (Shukla et al., 1990; Fearnside, 1995b).
Exponentiation of the present matrix of
transfer probabilities (Table VII) yields a vector representing the proportion
of land in each category after establishment of equilibrium (Jeffers, 1978:
92-97). Performing these calculations
indicates that the equilibrium landscape (Table VIII) would contain 0.0%
forest, 4.0% farmland, 43.8% productive pasture, 5.2% degraded pasture, 2.0%
secondary forest derived from farmland and 44.9% secondary forest derived from
pasture. In Table VIII a weighted
average is calculated of the biomass of vegetation in this equilibrium
landscape, resulting in a value of 28.5 t/ha.
(Tables VII and VIII here)
The above calculations refer only to land
that is cleared for agriculture and ranching.
Hydroelectric development also removes forest land.
4.2. Fate of
Biomass Carbon Stocks
The carbon stocks will change over a period
of years to approach those in the equilibrium landscape, with the quantities in
each pool increasing or decreasing at a different pace. The initial burn releases carbon immediately,
while subsequent burns do so over a period of about ten years. Bacterial decomposition and termite activity
also occurs largely over the first decade.
Soil carbon pools change relatively quickly at the surface, but
adjustments take longer for deeper pools (only carbon to 20 cm is considered in
the current calculation). Charcoal
(char) in the soil is a very long-term pool, considered to be permanently
sequestered in the analysis.
Charcoal formed in burning is one way that
carbon can be transferred to a long-term pool from which it cannot enter the
atmosphere. A burn of forest being
converted to cattle pasture near Manaus resulted in 2.7% of above-ground carbon
being converted to charcoal (Fearnside et al., 1993). Using the mean of this and additional studies
at Altamira and Manaus (Fearnside et al., nd-a,c) gives a mean of 1.9%
(Table IX). This is substantially lower
than the 15-23% assumed by Seiler and Crutzen (1980: 236) when they identified
charcoal formation as a potentially important carbon sink (more recent
calculations have used 5-10% charcoal yield: Crutzen and Andreae, 1990:
1672). Using the observed lower rate of
charcoal formation would make global carbon cycle models indicate a larger
contribution of greenhouse gases from tropical deforestation than has been the
case using the higher rates of carbon transfer to long-term pools (e.g.,
Goudriaan and Ketner, 1984).
The burning behavior of ranchers can alter
the amount of carbon passing into a long-term pool as charcoal. Carbon budget calculations often assume that
forest is burned only once, and that all unburned biomass subsequently
decomposes (e.g., Bogdonoff et al., 1985). This is not the typical pattern in cattle
pastures that dominate land use in deforested areas in the Brazilian Amazon. Ranchers reburn pastures at intervals of 2-3
years to combat invasion of inedible woody vegetation. Logs lying on the ground when these
reburnings occur are often burned. Some
charcoal formed in earlier burns can be expected to be combusted as well. Parameters for transformations of gross
carbon stocks are given in Table IX. A
typical scenario of three reburnings over a 10-year period would raise the
percentage of above-ground C converted to charcoal from 1.9% to 2.4% (Tables IX
and X). In addition, a small amount of
elemental carbon would be formed as graphitic particulates in the smoke (not
considered here); over 80% of the elemental carbon formed remains on the site
as charcoal (Kuhlbusch and Crutzen, 1995).
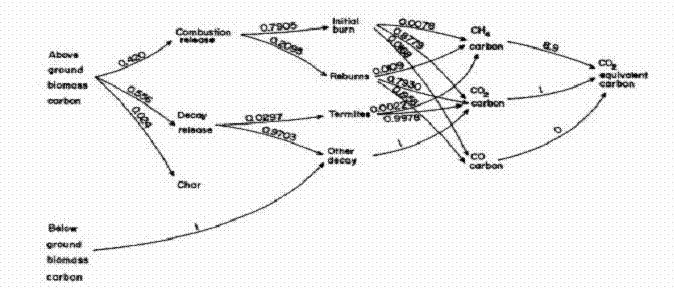
The carbon transformations over a typical 10-year sequence are shown
diagrammatically in Figure 3. These and
other calculations are carried out in a series of interlinked spreadsheets.
(Figure 3 and Tables IX and X here)
5. SOURCES AND SINKS
OF GREENHOUSE GASES
5.1. Burning
Biomass carbon not converted to charcoal
(or elemental C in the smoke) is released gradually through combustion and
decay, the relative importance of each affecting the gases emitted. If an area were burned only once, 33.2% of
the pre-burn above-ground carbon would be released through combustion and 64.8%
through decay (the remainder enters sinks as charcoal or graphitic
particulates) (Table IX). With a typical
scenario of three reburnings, 42.0% would be released through combustion and
55.6% through decay. Both combustion and
decay release other trace gases such as methane.
In these calculations, the burning
efficiency used for the initial burn (33.2%) is the mean of three studies
(Fearnside et al., 1993, nd-a,b), adjusted for the effect of removal of
trunks by logging (Fearnside, nd-b). The
burning studies used found, respectively, efficiencies of 27.6% in a 1984 burn
near Manaus, Amazonas, 42.0% as the mean of three 1986 burns near Altamira,
Pará, and 28.3% in a 1990 burn near Manaus.
Kauffman et al. (1995) have recently found higher burning
efficiencies: 51.5% in a 1990 burn at Jacundá, Pará, 51.3% in a 1991 burn at
Marabá, Pará, 40.5% in a 1992 burn at Santa Barbara, Rondônia, and 56.1% in a
1992 burn at Jamurí, Rondônia.
Parameters for carbon emissions (CO2,
CH4 and CO) from the different burning and decay transformations of
biomass are given in Table X. Two sets
of parameters are given: a "low trace gas" and a "high trace
gas" scenario, reflecting the range of values appearing in the literature
for trace gas releases from such sources as termites and flaming and smoldering
burns. Carbon emissions as CO2,
CH4 and CO are diagrammed in Figure 4 with parameters for the low
trace gas scenario. The low and high
trace gas scenarios reflect the range of values for trace gas emissions only,
not the level of uncertainty for factors affecting the amount of material that
burns or decays, such as forest biomass, deforestation rate and burning
efficiency. Parameters for other sources
of greenhouse gases from land-use change are given in Table XI, and trace gas
release parameters are given in Table XII.
(Figure 4 and Tables XI and XII here)
The amount of methane released is heavily
dependent on the ratio of smoldering to flaming combustion; smoldering releases
substantially more CH4.
Aircraft sampling over fires (mostly from virgin forest clearing)
indicates that a substantial fraction of combustion is in smoldering form
(Andreae et al., 1988). Logs
consumed by reburning of cattle pastures are virtually all burned through
smoldering rather than flaming combustion (personal observation).
Carbon monoxide (CO) is also produced by
burning. This gas contributes indirectly
to the greenhouse effect by impeding natural cleansing processes in the
atmosphere that remove a number of greenhouse gases, including methane. Carbon monoxide removes hydroxyl radicals
(OH), which react with CH4 and other gases.
Burning also releases some nitrous oxide (N2O),
which contributes both to the greenhouse effect and to the degradation of
stratospheric ozone. A sampling artifact
has made measurements prior to 1989 unusable (Muzio and Kramlich, 1988). Estimates after discovery of the artifact
indicate N2O emissions from biomass burning are substantially lower
than had previously been thought (Crutzen and Andreae, 1990). Parameters used in the present estimate
(Table XII) are unaffected by the artifact.
5.2. Removal of
Intact Forest Sources and Sinks
Deforestation makes an additional
contribution to methane by removing a CH4 sink in the soil of intact
forest (Table XII). Removal of intact
forest sources and sinks also affect the contribution of deforestation to a
variety of compounds of nitrogen and oxygen (NOx) and to non-methane
hydrocarbons (NMHC), especially isoprenes.
Effects of removing intact forest sources are included in the parameters
for trace gases (Table XII). No forest
sink is explicitly included for N2O because the emission values used
for this gas represent the net difference between forest and pasture emissions.
In the case of NMHC, the net effect of
deforestation is to decrease the source strength over the 100-year period used
in the current calculation. The
magnitude of the effect of this reduction on global warming cannot be
calculated yet. Indirect effects of
trace gases were included in the 1990 IPCC scientific assessment of climate
change (J.T. Houghton et al., 1990) but were dropped in the 1992 IPCC
supplementary report pending resolution of disagreements over the magnitude of
the effects (J.T. Houghton et al., 1992). Some of the indirect effects were restored in
the 1994 report in the case of CH4, raising its 100-year time
horizon global warming potential from 11 to 24.5 as compared to CO2
on a mass basis (Albritton et al., 1995: 222), making 1 t of CH4
carbon equivalent to 8.9 t of CO2 carbon [Note these values are
expected to decrease to 21 and 7.6, respectively, in the 1995 IPCC
report]. No indirect effects are yet
agreed upon for NOx and NMHC (as well as CO), which continue to be
considered as having zero global warming potential (Albritton et al.,
1995: 222).
5.3. Soil Carbon
Release of soil carbon would be expected
when forest is converted to pasture because soil temperatures increase when
forest cover is removed, thus shifting the balance between organic carbon
formation and degradation to a lower equilibrium level (Cunningham, 1963; Nye
and Greenland, 1960). A number of
studies have found lower carbon stocks under pasture than forest (reviewed in
Fearnside, 1980). For the same reason,
naturally occurring tropical grasslands also have much smaller soil carbon
stocks per hectare than do forests (Post et al., 1982). Lugo et al. (1986), however, have
found increases in carbon storage in pasture soils in Puerto Rico, especially
in drier sites, and suggest that tropical pastures may be a carbon sink. The present study treats soils as a source of
carbon when forests are converted to pasture.
All carbon released from soils is assumed to be in the form of CO2.
Soil carbon in pasture is taken to be that
in a profile equivalent to what is compacted from a 20-cm profile in the
forest. Parameters used in deriving soil
carbon changes are given in Table XIII.
The layer compacted from the top 20 cm of forest soil releases 3.92 t/ha
of carbon (the value used in the current calculations).
(Table XIII here)
The 3.92 t/ha release from the top 20 cm of
soil represents 38% of the pre-conversion carbon present in this layer. This is higher than the 20% of pre-conversion
carbon in the top 40 cm of soil that Detwiler (1986) concluded is released, on
average, from conversion to pasture. The
difference is not so great as it might seem: since carbon release is greatest
nearest the surface, considering soil to 40 cm would thereby reduce the
percentage released. One factor acting
to compensate for any overestimation possibly caused by using a higher
percentage of soil carbon release in the present study is the low bias
introduced by having considered only the top 20 cm.
If soil to one-m depth were considered (the
usual practice), and the same 38% of pre-conversion carbon were assumed to be
released, then the release would be increased to 9.33 t/ha (Table XIII). The calculation to one-m depth considers that
the top 20 cm of soil contains 42% of the carbon in a one-m profile (based on
samples near Manaus: Fearnside, 1987).
Brown and Lugo (1982: 183) have used a similar relationship to estimate
carbon stocks in Thailand to a depth of one m from samples of the top 20 cm,
considering 45% of the carbon in a one-m profile to be located in the top 20
cm.
Soil carbon release may be substantially
higher than the values used here.
Nepstad et al. (1994) have found carbon stocks 16 t/ha lower in
the top one m of soil under degraded pasture as compared to intact forest,
implying a carbon emission from soil about twice the values used. The combined effect of adopting these higher
values and extending consideration from the top 20 cm to the top meter of soil
would be to increase soil emissions by a factor of four, resulting in an
additional net committed emission of about 30 X 106 t of CO2-equivalent
carbon from the area cleared in 1990.
On the other hand, some studies have
indicated less carbon loss than the value adopted here. Desjardins et al. (1994) found a net
loss of 2.3 t/ha in the top 20 cm of soil in Pará, about 40% less than the
value used here. The authors of the
study believed that carbon loss in the 10-year-old pasture was relatively low
because it had not been actively used "for a few years" (Desjardins et
al., 1994: 112). Cerri et al.
(1991: 254) found that 12 t/ha were lost from the top 20 cm during the first
year, but that 8-year-old pasture had recovered the soil carbon stocks of
forest and increased the stock in the top 20 cm to a level 6 t/ha above that in
the forest soil. They attributed the
strong recovery to "a rather exceptional management" in an
"almost ideal well managed pasture" at an agricultural experiment
station near Manaus (Cerri et al., 1991: 255). The studies indicating only slight carbon
loss or a carbon gain do not include correction for soil compaction under
pasture, making them underestimate the carbon loss that occurs from the layer
of soil compacted from a given layer of the original forest soil (Fearnside,
1985, 1987; Veldkamp, 1993).
Soil below one-m depth may also be
releasing significant amounts of carbon in deforested areas. Nepstad et al. (1994) have found
carbon stocks at 1-8 m depth exceeding those in the top meter, and isotopic
data indicate that this deeper pool experiences significant turnover, which is
expected to result in carbon releases as the elimination of deep roots removes
the source of replenishment for the deep soil carbon pool. Major adjustments of soil carbon inventory,
including pools in the deeper soil layers, occur within the first decade after
deforestation (Trumbore et al., 1995).
The present analyis considers all loss of
carbon inventory from the top 20 cm of forest soil to be emissions, whether or
not the losses occur through release of CO2 directly to the
atmosphere or by leaching to deeper soil layers and from there to groundwater
and river outflow. The atmospheric
effect of loss to groundwater is probably almost the same as if the carbon had
been released directly. Since carbon in
groundwater is in dissolved rather than particulate form, it would not enter
ocean sediments and would remain exposed to oxidation after reaching the sea. Half of the carbon entering the Amazon River
is oxidized before it reaches the ocean (Richey et al., 1980:
1350). A modest amount of carbon is
undoubtedly lost through erosion as particulates and survives transport to
long-term pools in ocean sediments (not considered in the current analysis).
5.4. Termites and
Decay
A
lively controversy surrounds the question of how much methane is produced by
termites (Collins and Wood, 1984; Fraser et al., 1986; Rasmussen and
Khalil, 1983; Zimmerman et al., 1982, 1984). This stems from differing estimates of the
number of termites, the amount of wood consumed per termite, and the methane
emission per gram of wood consumed. No
measurement exists of the percentage of felled biomass that is ingested by
termites in Amazonian clearings. Termite
populations increase to a peak approximately 5-6 years after clearing, and
subsequently decline as the available wood disappears (A.G. Bandeira, personal
communication, 1990). It is assumed that
none of the below-ground wood is ingested by termites: a conservative assumption
given that termite species that eat buried wood are known to occur (Bandeira
and Macambira, 1988) and termites consume underground biomass in other regions,
such as Africa (e.g. Wood et al., 1977).
The
termite emissions are limited by the ability of the termite populations to
expand to consume the large amount of dead wood biomass that becomes available
to them after deforestation. Both in the
forest and in clearings, the inability of the observed populations to ingest
the quantity of wood available makes the amount of wood biomass consumed
independent of the amount of wood present.
The calculations used here (Martius et al., nd) are based on the
one available measurement of wood consumption, which is for the termite Nasutitermes
macrocephalus (a wood-feeding species in the Amazonian várzea): 49
mg of wood dry weight consumption per gram of termite biomass per day (Martius,
1989: 228).
Both the high and low trace gas scenarios
use the average emission rate for wood-feeders (Martius, et al., 1993)
applied to the total termite biomass of wood-, soil- and leaf-feeders. This emission of 3.01.3 μg/hour is equivalent to 0.0022 tons
of carbon released as methane per ton of carbon consumed (considering 50%
carbon content of wood). This is virtually
identical to the value obtained by Seiler et al. (1984) for termites in
Africa. It is lower by a factor of four
than the 0.0079 t methane C released/t wood C consumed estimated for Amazonian
termites by Goreau and de Mello (1987).
However, the lower value (Martius, et al., 1993) is believed to
be more reliable, as the higher value was based on monitoring a single nest for
only two days, whereas the value used here is based on monitoring 15 nests for
two years.
5.5. Cattle and
Pasture
Methane is produced in the rumens of cattle
that occupy pastures in deforested areas.
The portion of the deforested area considered to be maintained under
pasture is that derived from the equilibrium landscape (Tables VII and VIII). Parameters used to derive methane emissions
from cattle are included in Table XI.
Pasture soils in Amazonia emit N2O
in quantities substantially higher than forest soils when measurements are made
over a full annual cycle (Luizão et al., 1989). Most emissions are in the wet season, and are
not reflected in measurements restricted to the dry season (e.g. Goreau and de
Mello, 1987). Unlike emissions from the
initial burning, conversion of a given hectare to pasture does not result in a
one-time release of this greenhouse gas, but rather a continuous additional
flux at this rate for as long as the area is maintained under this land use
(the assumption in the present calculation).
However, Keller et al. (1993) have found that N2O
emissions from pasture soil declines with pasture age in Costa Rica, falling
below emission levels from forest soils after 10 years.
One factor not included in the calculation
is production of trace gases by reburning of pasture and secondary forest. Combustion of logs remaining from the
original forest is included. Burning of
the biomass of the pasture itself and of secondary forest does not contribute
to net release of carbon dioxide, as the same amount of carbon is reabsorbed
when the vegetation regrows. However, CH4,
CO, N2O and NOx do increase as a result of reburnings as
these gases do not enter photosynthetic reactions. Methane degrades to CO2 after an
average of 14.5 years (Albritton et al., 1995: 222), and CO degrades
after a few months (Thompson and Cicerone, 1986: 10,857), after which the
carbon can return to the vegetation. The
trace gas inputs of reburning the replacement vegetation represent one of
several factors not included in the current calculation of net committed
emissions, but which are included in calculations of the annual balance of emissions
(Fearnside, in press-b). Burning of cerrado
and other savanna vegetation without clearing of trees is a source of trace gas
emissions in the annual balance, but does not contribute to net committed
emissions (because only the fate of the cleared area is followed).
5.6. Hydroelectric
Dams
Because no new reservoirs were filled in
1990, the calculations presented above consider only emissions from conversion
of natural forest vegetation to a landscape dominated by cattle pasture--the
dominant trend in Brazilian Amazonia today.
Hydroelectric dams in rainforest areas release greenhouse gases both by
decomposition of dead forest left standing in the reservoirs and by the
continuing release of methane from flooded areas (especially in portions that
are alternately dried and flooded).
Although hydroelectric dams are commonly
believed to have no impact on the greenhouse effect, in contrast to fossil fuel
use, the validity of this conclusion depends heavily on the biomass of the
vegetation in the flooded areas and on the power output of the dams. In Amazonia, dams are sometimes worse than
petroleum from the point of view of greenhouse gas emissions. The worst case is Balbina Dam, which was
closed in 1987. Located on relatively
flat terrain, Balbina's shallow 3147 km2 reservoir can generate only
enough power to deliver annually an average of 109 megawatts to Manaus
(Fearnside, 1989a). The biomass of the
flooded forest is now decomposing, releasing its carbon to the atmosphere. Methane is also released from decay of
biomass under the anoxic conditions at the bottom of the reservoir. Generating the same energy from petroleum
would take 250 years to equal the carbon release from flooding the Balbina
reservoir (based on Junk and Nunes de Mello, 1987; see Fearnside, 1989a). However, most of the carbon release from
reservoirs takes place in the first decade, which increases the importance of
these emissions relative to those from fossil fuels when calculations include time
horizons and/or weighting of emissions for time preference as by
discounting. In 1990, Balbina was
releasing twenty times more CO2-equivalent carbon than would have
been produced by generating the same power from fossil fuels (Fearnside,
1995c).
Calculating emissions for hydroelectric dams
requires estimating vertical distribution of the biomass in order to calculate
releases occurring above the water, in the seasonally-inundated zone, and
underwater in the surface and anoxic water portions of the reservoir. Transfers from the above-water to the
underwater categories occur as branches and trunks fall. Assumptions about decay rates and paths allow
estimation of emissions of CO2 and CH4 from forest
biomass. To these emissions are added CH4
releases from decay of macrophytes and of organic matter entering the reservoir
from the river. The emission in 1990
from the approximately 5500 km2 of reservoirs in the region was 38 X
106 t of CO2 gas and 0.22 X 106 t of CH4
gas (Fearnside, 1995c). In 1990, no new
reservoirs were filled in the Legal Amazon.
Amazonian várzea (white water
floodplain) has been identified as one of the world's major sources of
atmospheric methane (Mooney et al., 1987). Várzea occupies about 2% of the five X
106 km2 Legal Amazon, the same percentage that would be
flooded if all of the reservoirs planned for the region are created. A total of 100,000 km2 would be
flooded (Brazil, ELETROBRÁS, 1987: 150), or about 20 times the present
reservoir area. Virtually all planned
hydroelectric dams are in the forested portion of the region--and the
reservoirs would flood approximately 2.5% of this portion of the region. Were these reservoirs to contribute an output
of methane per hectare on the same order as that produced by the várzea,
they would together represent a significant contribution to the greenhouse
effect. The annual emission of methane
to the atmosphere from the várzea has been calculated by Devol et al.
(1990) to be 5.1 X 106 t CH4/year. Like biogenic release of N2O, this
would be a permanent addition to greenhouse gas sources, rather than a one-time
input. Unfortunately, CH4
emission measurements from Amazonian reservoirs do not yet exist, making
measurements in várzea lakes the best available surrogate.
6. NET COMMITTED
EMISSIONS
The quantities of gases released by each
source and absorbed by each sink in the 13.8 X 103 km2 of
forest and 5 X 103 km2 of cerrado cleared in 1990
are given in Table XIV for the low trace gas scenario. Table XV presents the corresponding results
for the high trace gas scenario.
Although the emissions of CO2 dwarf the absolute quantities
of the other gases, the greater greenhouse impact per ton of the latter gives
them a significant role in deforestation's contribution to global warming.
(Tables XIV and XV here)
I have converted my estimated emissions of
trace gases to CO2 equivalents (Table XVI). The conversion uses the global warming
potentials adopted by the Intergovernmental Panel for Climate Change (IPCC) in
its 1994 report (Albritton et al., 1995: 222). These express the impact on global warming of
an immediate emission of one ton of each gas relative to emitting one ton of CO2
gas, considering a 100-year time horizon without discounting. The exclusion of a number of indirect
effects, the long time horizon and the lack of discounting all reduce the
weight given to CH4 and CO relative to CO2, thereby
reducing the calculated impact of tropical deforestation relative to fossil
fuel combustion (Fearnside, 1992a).
(Table XVI here)
Using this conservative methodology for
expressing trace gas impact, the CO2-equivalent carbon from trace
gases represents 5% and 9% of the total in the low and high trace gas
scenarios, respectively. The total
contribution of 1990 clearing in forest and cerrado was 274-285 X 106
t of CO2-equivalent carbon (Table XVI). In addition to this, logging in 1990 emitted
61 X 106 t CO2-equivalent C (Fearnside, nd-b). As this was calculated assuming a constant
logging harvest at the 1988 official rate (24.6 X 106 m3/year),
it reflects net committed emissions from logging. Adding this to the total from forest clearing
raises the net committed emission for 1990 land-use change in the Brazilian
Legal Amazon to 335-346 X 106 t CO2-equivalent
carbon. This represents roughly 5% of
the total global emissions from deforestation and fossil fuel sources,
indicating the high impact of deforestation.
At the same time, it makes clear the fact that reducing or halting
deforestation is not enough to avoid the most damaging consequences of global
warming, and that use of fossil fuels must also be substantially reduced.
* NOTE
(*) Some inconsistency
remains in the definition of original forest area used here (Tables III and
IV), and that used in the deforestation estimate (Tables I and II). The deforestation estimate used a line
between forest and nonforest drawn by INPE from LANDSAT-TM 1:250,000 scale
images with some reference to the RADAMBRASIL vegetation maps (but without a
list of vegetation types classified as forest and nonforest). The area so defined has not yet been measured
by INPE, but a compilation by map sheet (using IBGE 1:250,000 scale maps as a
geographical base) was made of the approximate proportions of forest and
nonforest in each sheet. The total from
this compilation is 4.0 X 106 km2, lower than the 4.3 X
106 km2 measured from the IBGE/IBDF 1:5,000,000 scale
map.
The "present" vegetation is also
inconsistent: the IBGE/IBDF mapping totals 3.7 X 106 km2
of forest (circa 1988) (Table IV), whereas the original forest area from the
same map, less the area deforested by 1988 (Table I), yields a total of 3.9 X
106 km2.
ACKNOWLEDGMENTS
Studies on burning in Altamira were funded
by National Science Foundation grants GS-422869 (1974-1976) and ATM-86-0921
(1986-1988), in Manaus by World Wildlife Fund-US grant US-331 (1983-1985), and
in Manaus and Roraima by the Pew Scholars Program in Conservation and the
Environment and by the Fundação Banco do Brasil (Grant 10/1516-2). I thank P. Crutzen, C.A.S. Hall, O. Masera,
G.H. Platais and S.V. Wilson for comments.
REFERENCES
Ahuja, D.R.: 1990,
`Regional anthropogenic emissions of greenhouse gases', in Intergovernmental
Panel on Climate Change (IPCC), Response Strategies Working Group (RSWG),
Subgroup on Agriculture, Forestry and other Human Activities (AFOS), Proceedings
of the Conference on Tropical Forestry Response Options to Global Climate
Change, U.S. Environmental Protection Agency, Office of Policy Assessment
(USEPA-OPA, PM221), Washington, DC, 417-461.
Albritton, D.L.,
Derwent, R.G., Isaksen, I.S.A., Lal, M. and Wuebbles, D.J.: 1995, 'Trace gas
radiative forcing indices' in Houghton, J.T., Meira Filho, L.G., Bruce, J.,
Hoesung Lee, Callander, B.A., Haites,
E., Harris N. and Maskell, K. (eds.) Climate Change 1994: Radiative Forcing
of Climate Change and An Evaluation of the IPCC IS92 Emission Scenarios,
Cambridge University Press, Cambridge, UK, 205-231.
Andreae, M.O., Browell, E.V., Garstang, M., Gregory, G.L., Harriss, R.C., Hill, G.F., Jacob, D.J., Pereira, M.C., Sachse, G.W., Setzer, A.W., Silva Dias, P.L., Talbot, R.W., Torres, A.L., and Wofsy, S.C.: 1988, `Biomass-burning emissions and associated haze layers over Amazonia', J. Geophys. Res. 93(D2): 1509-1527.
Bandeira, A.G. and Macambira, M.L.J.: 1988, `Térmitas de Carajás, Estado do Pará, Brasil: Composição faunística, distribuição e hábito alimentar', Boletim do Museu Paraense Emílio Goeldi: Zoologia 4(2): 175-190.
Barbosa, R.I.: 1994, Efeito Estufa na Amazônia: Estimativa da Biomassa e a Quantificação do Estoque e Liberação de Carbono na Queima de Pastagens Convertidas de Florestas Tropicais em Roraima, Brasil, Masters thesis in ecology, Instituto Nacional de Pesquisas da Amazônia (INPA) and Universidade Federal do Amazonas (UFAM), Manaus, Brazil.
Bogdonoff, P., Detwiler, R.P., and Hall, C.A.S.: 1985, `Land use change and carbon exchange in the tropics: III. Structure, basic equations, and sensitivity analysis of the model', Environ. Manage. 9(4): 345-354.
Brazil, Instituto Brasileiro de Geografia e Estatística (IBGE) and Instituto Brasileiro de Desenvolvimento Florestal (IBDF): 1988, Mapa de Vegetação do Brasil, Map Scale 1:5,000,000, IBAMA, Brasilia.
Brazil, Instituto Nacional de Pesquisas Espaciais (INPE): 1992, Deforestation in Brazilian Amazonia. INPE, São José dos Campos, São Paulo, 3 pp.
Brazil, Ministério das Minas e Energia, Centrais Elétricas do Brasil (ELETROBRÁS): 1987, Plano 2010: Relatório Geral. Plano Nacional de Energia Elétrica 1987/2010. (Dezembro de 1987). ELETROBRÁS, Brasilia.
Brazil, Ministério das Minas e Energia, Departamento Nacional de Produção Mineral (DNPM), Projeto RADAMBRASIL: 1973-1983, Levantamento de Recursos Naturais, Vols. 1-23, DNPM, Rio de Janeiro.
Brown, S. and Lugo,
A.E.: 1982, `The storage and production of organic matter in tropical forests
and their role in the global carbon cycle', Biotropica 14(3):
161-187.
Brown, S. and Lugo,
A.E.: 1984, `Biomass of tropical forests: A new estimate based on forest
volumes', Science 223: 1290-1293.
Brown, S. and Lugo,
A.E.: 1992, 'Aboveground biomass estimates for tropical moist forests of the
Brazilian Amazon', Interciencia 17(1): 8-18.
Brown, S., Gillespie,
A.J.R., and Lugo, A.E.: 1989, `Biomass estimation methods for tropical forests
with applications to forest inventory data', For. Sci. 35(4):
881-902.
Buschbacher, R.J.:
1984, Changes in Productivity and Nutrient Cycling following Conversion of
Amazon Rainforest to Pasture, Ph.D. thesis, University of Georgia, Athens,
GA.
Cerri, C.C., Volkoff,
B., and Andreux, F.: 1991, `Nature and behaviour of organic matter in soils
under natural forest, and after deforestation, burning and cultivation, near
Manaus', For. Ecol. Manage. 38: 247-257.
Cofer, W.R., Levine,
J.S., Riggan, P.H., Sebacher, D.I., Winstead, E.L., Shaw, E.F., Brass, J.A.,
and Ambrosia, V.G.: 1988, `Trace gas emissions from a mid-latitude prescribed
chaparral fire', J. Geophys. Res. 93: 1653-1658.
Collins, N.M. and
Wood, T.G.: 1984, `Termites and atmospheric gas production', Science 224:
84-85.
Crutzen, P.J. and
Andreae, M.O.: 1990, `Biomass burning in the tropics: Impact on atmospheric
chemistry and biogeochemical cycles', Science 250: 1669-1678.
Crutzen, P.J.,
Aselmann, I., and Seiler, W.: 1986, `Methane production by domestic animals,
wild ruminants, other herbivorous fauna, and humans', Tellus 38B:
271-284.
Crutzen, P.J., Delany,
A.C., Greenberg, J., Haagenson, P., Heidt, L., Lueb, R., Pollock, W., Seiler,
W., Wartburg, A., and Zimmerman, P.: 1985, `Tropospheric chemical composition
measurements in Brazil during the dry season', J. Atm. Chem. 2:
233-256.
Cunningham, R.H.:
1963, `The effect of clearing a tropical forest soil', J. Soil Sci. 14:
334-344.
Desjardins, T.,
Andreux, F., Volkoff, B., and Cerri, C.C.: 1994, `Organic carbon and 13C contents
in soils and soil size-fractions, and their changes due to deforestation and
pasture installation in eastern Amazonia', Geoderma 61: 103-118.
Detwiler, R.P.: 1986,
`Land use change and the global carbon cycle: The role of tropical soils', Biogeochem.
2: 67-93.
Detwiler, R.P. and
Hall, C.A.S.: 1988, `Tropical forests and the global carbon cycle', Science
239: 42-47.
Devol, A.H., Richey,
J.H., Forsberg, B.R., and Martinelli, L.A.: 1990, `Seasonal dynamics in methane
emissions from the Amazon River floodplain to the troposphere', J. Geophys.
Res. 95(D10): 16,417-16,426.
Dickinson, R.E. and
Henderson-Sellers, A.: 1988, `Modelling tropical deforestation: A study of GCM
land-surface parameterizations', Quar. J. Royal Meteorolog. Soc. 114:
439-462.
dos Santos, J.R.: 1989, `Estimativa da biomassa foliar das savanas brasileiras: Uma abordagem por sensoriamento remoto', in IV Simpósio Latinamericano en Percepción Remota, IX Reunión plenaria SELPER, 19 al 24 de noviembre de 1989, Bariloche, Argentina. Tomo 1: 190-199.
Falesi, I.C.: 1976, Ecossistema de Pastagem Cultivada na Amazônia Brasileira, Centro de Pesquisa Agropecuária do Trópico Úmido (CPATU), Belém, Para.
Fearnside, P.M.: 1979,
`Cattle yield prediction for the Transamazon Highway of Brazil', Interciencia
4(4): 220‑225.
Fearnside, P.M.: 1980,
`The effects of cattle pasture on soil fertility in the Brazilian Amazon:
Consequences for beef production sustainability', Trop. Ecol. 21(1):
125-137.
Fearnside, P.M.: 1985,
`Brazil's Amazon forest and the global carbon problem', Interciencia 10(4):
179‑186.
Fearnside, P.M.: 1987,
`Summary of progress in quantifying the potential contribution of Amazonian
deforestation to the global carbon problem', in Athié, D., Lovejoy, T.E., and
Oyens, P. de M., (eds.) Proceedings of the Workshop on Biogeochemistry of
Tropical Rain Forests: Problems for Research, Universidade de São Paulo,
Centro de Energia Nuclear na Agricultura (CENA), Piracicaba, São Paulo, 75-82.
Fearnside, P.M.: 1989a, `Brazil's Balbina Dam: Environment versus the legacy of the pharaohs in Amazonia', Environ. Manage. 13(4): 401-423.
Fearnside, P.M.: 1989b, A Ocupação Humana de Rondônia: Impactos, Limites e Planejamento (CNPq Relatórios de Pesquisa No. 5), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Brasilia.
Fearnside, P.M.: 1990,
`The rate and extent of deforestation in Brazilian Amazonia', Environ. Cons.
17(3): 213-216.
Fearnside, P.M.: 1991,
`Greenhouse gas contributions from deforestation in Brazilian Amazonia', in
Levine, J.S. (ed.) Global Biomass Burning: Atmospheric, Climatic, and
Biospheric Implications, MIT Press, Cambridge, MA, 92-105.
Fearnside, P.M.:
1992a, Greenhouse Gas Emissions from Deforestation in the Brazilian Amazon,
Carbon Emissions and Sequestration in Forests: Case Studies from Developing
Countries, Volume 2, LBL-32758, UC-402, Climate Change Division, Environmental
Protection Agency, Washington, DC and Energy and Environment Division, Lawrence
Berkeley Laboratory (LBL), University of California (UC), Berkeley, CA.
Fearnside, P.M.:
1992b, `Forest biomass in Brazilian Amazonia: Comments on the estimate by Brown
and Lugo', Interciencia 17(1): 19-27.
Fearnside, P.M.:
1993a, `Deforestation in Brazilian Amazonia: The effect of population and land
tenure', Ambio 22(8): 537-545.
Fearnside, P.M.: 1993b, `Desmatamento na Amazônia: Quem tem razão --o INPE ou a NASA?', Ciência Hoje 16(96): 6-8.
Fearnside, P.M.:
1993c, `Biomass of Brazil's Amazonian forests: Reply to Brown and Lugo
revisited', Interciencia 18(1): 5-7.
Fearnside, P.M.:
1995a, `Global warming response options in Brazil's forest sector: Comparison
of project-level costs and benefits', Biomass and Bioenergy 8(5):
309-322.
Fearnside, P.M.:
1995b, `Potential impacts of climatic change on natural forests and forestry in
Brazilian Amazonia', For. Ecol. Manage. 78: 51-70.
Fearnside, P.M.:
1995c, `Hydroelectric dams in the Brazilian Amazon as sources of greenhouse
gases', Environ. Cons. 22(1): 7-19.
Fearnside, P.M.: 1996,
`Amazonian deforestation and global warming: Carbon stocks in vegetation
replacing Brazil's Amazon forest', For. Ecol. Manage. 80, 21-34.
Fearnside, P.M.: in
press-b, `Amazonia and global warming: Annual balance of greenhouse gas
emissions from land-use change in Brazil's Amazon region', in Levine, J. (ed.) Biomass
Burning and Global Change, MIT Press, Cambridge, MA.
Fearnside, P.M.: nd-a,
`Biomass of Brazil's Amazonian forests', (in preparation).
Fearnside, P.M.: nd-b,
'Tropical forest logging and management:
Implications for global warming', (in preparation).
Fearnside, P.M. and
Ferraz, J.: 1995, `A conservation gap analysis of Brazil's Amazonian
vegetation', Cons. Biol. 9(5): 1134-1147.
Fearnside, P.M. and Guimarães, W.M.: 1996, `Carbon uptake by secondary forests in Brazilian Amazonia', For. Ecol. Manage. 80, 35-46.
Fearnside, P.M., Tardin, A.T. and Meira Filho, L.G.: 1990, Deforestation rate in Brazilian Amazonia, Instituto de Pesquisas Espaciais (INPE), São José dos Campos, São Paulo, 8 pp.
Fearnside, P.M., Leal
Filho, N., and Fernandes, F.M.: 1993, `Rainforest burning and the global carbon
budget: Biomass, combustion efficiency and charcoal formation in the Brazilian
Amazon', J. Geophys. Res. 98(D9): 16,733-16,743.
Fearnside, P.M.,
Graça, P.M.L.A., Leal Filho, N., Rodrigues, F.J.A., and Robinson, J.M.: nd-a,
`Tropical forest burning in Brazilian Amazonia: Measurements of biomass,
burning efficiency and charcoal formation at Altamira, Pará', (in preparation).
Fearnside, P.M., Leal
Filho, N., Graça, P.M.L.A., Ferreira, G.L., Custodio, R.A., and Rodrigues,
F.J.A.: nd-b, `Pasture biomass and productivity in Brazilian Amazonia', (in
preparation).
Fearnside, P.M.,
Barbosa, R.I., and Graça, P.M.L.A.: nd-c,
`Burning of secondary forest in Amazonia: Biomass, burning efficiency
and charcoal formation during land preparation for agriculture in Roraima,
Brazil', (in preparation).
Fearnside, P.M.,
Graça, P.M.L.A., and Rodrigues, F.J.A.: nd-d,
`Biomass, combustion efficiency and charcoal formation in Amazonia:
Measurements in a rainforest burn near Manaus, Brazil', (in preparation).
Fraser, P.J.,
Rasmussen, R.A., Creffield, J.W., French, J.R., and Khalil, M.A.K.: 1986,
`Termites and global methane--another assessment', J. Atmos. Chem. 4:
295-310.
Glerum, B.B.: 1960,
Report to the Government of Brazil on a Forestry Inventory in the Amazon Valley
(Part Five) (Region between Rio Caete and Rio Maracassume), FAO Report No.
1250, Project No. BRA/FO, Food and Agriculture Organization of the United
Nations (FAO), Rome, 67 pp.
Goreau, T.J. and de
Mello, W.Z.: 1987, `Effects of deforestation on sources and sinks of
atmospheric carbon dioxide, nitrous oxide, and methane from central Amazonian
soils and biota during the dry season: A preliminary study', in Athié, D.,
Lovejoy, T.E., and Oyens, P. de M., (eds.) Proceedings of the Workshop on
Biogeochemistry of Tropical Rain Forests: Problems for Research,
Universidade de São Paulo, Centro de Energia Nuclear na Agricultura (CENA),
Piracicaba, São Paulo, 51-66.
Goudriaan, J. and
Ketner, P.: 1984, `A simulation study for the global carbon cycle, including
man's impact on the biosphere, Clim. Change 6: 167-192.
Greenberg, J.P., Zimmerman, P.R., Heidt, L., and Pollock, W.: 1984, `Hydrocarbon and carbon monoxide emissions from biomass burning in Brazil', J. Geophys. Res. 89(D1): 1350-1354.
Guimarães, W.M.: 1993, Capoeira em Pastagens Degradadas e o Efeito Estufa: Quantificação do Papel da Vegetação Secundária no Balanço de Carbono Resultante do Desmatamento na Amazônia Brasileira, Masters thesis, Instituto Nacional de Pesquisas da Amazônia/Fundação Universidade do Amazonas (INPA/FUA), Manaus.
Hall, C.A.S., and
Uhlig, J.: 1991, `Refining estimates of carbon released from tropical land-use
change', Can. J. For. Res. 21: 118-131.
Hecht, S.B.: 1981,
`Deforestation in the Amazon Basin: Magnitude, dynamics, and soil resource
effects', Studies in Third World Societies No. 13: 61-108.
Heinsdijk, D.: 1957,
Report to the Government of Brazil on a Forest Inventory in the Amazon Valley
(Region between Rio Tapajós and Rio Xingú), FAO Report No. 601, Project No. BRA/FO,
Food and Agriculture Organization of the United Nations (FAO), Rome, 135 pp.
Heinsdijk, D.: 1958a,
Report to the Government of Brazil on a Forestry Inventory in the Amazon Valley
(Part Two) (Region between Rio Xingú and Rio Tocantins), FAO Report No. 949,
Project No. BRA/FO, Food and Agriculture Organization of the United Nations
(FAO), Rome, 83 pp.
Heinsdijk, D.: 1958b,
Report to the Government of Brazil on a Forest Inventory in the Amazon Valley
(Part Three) (Region between Rio Tapajós and Rio Madeira), FAO Report No. 969,
Project No. BRA/FO, Food and Agriculture Organization of the United Nations
(FAO), Rome, 72 pp.
Heinsdijk, D.: 1958c,
Report to the Government of Brazil on a Forest Inventory in the Amazon Valley
(Part Four) (Region between Rio Tocantins and Rios Guamá and Capim), FAO Report
No. 992, Project No. BRA/FO, Food and Agriculture Organization of the United
Nations (FAO), Rome, 94 pp.
Houghton, J.T.,
Callander, B.A., and Varney, S.K. (eds.): 1992,
Climate Change 1992: The Supplementary Report to the IPCC Scientific
Assessment, Cambridge University Press, Cambridge, UK.
Houghton, J.T.,
Jenkins, G.J., and Ephraums, J.J. (eds.): 1990, Climate Change: The IPCC
Scientific Assessment, Cambridge University Press, Cambridge, UK.
Houghton, R.A.: 1991,
`Tropical deforestation and atmospheric carbon dioxide', Clim. Change 19(1-2):
99-118.
Jeffers, J.N.R.:
1978, An Introduction to Systems
Analysis: With Ecological Applications, Arnold, London.
Junk, W.J. and Nunes de Mello, J.A.S.: 1987, `Impactos ecológicos das represas hidrelétricas na bacia amazônica brasileira', in Kohlhepp, G. and Schrader, A., (eds.) Homem e Natureza na Amazônia, Tübinger Geographische Studien 95 (Tübinger Beiträge zur Geographischen Lateinamerika-Forschung 3), Geographisches Institut, Universität Tübingen, Tübingen, Germany, 367‑385.
Kaplan, W.A., Wofsy,
S.C., Keller, M., and da Costa, J.M.: 1988,
`Emission of NO and deposition of O3 in a tropical forest
system', J. Geophys. Res. 93: 1389-1395.
Kauffman, J.B., Cummings,
D.L., Ward, D.E. and Babbitt, R.: 1995, `Fire in the Brazilian Amazon: Biomass,
nutrient pools, and losses in slashed primary forests', Oecologia 104:
397-408.
Kaufman, Y.J., Setzer,
A.W., Justice, C., Tucker, C.J., Pereira, M.G., and Fung, I.: 1990, `Remote
sensing of biomass burning in the tropics', in Goldammer, J.G., (ed.), Fire
in the Tropical Biota: Ecosystem Processes and Global Challenges,
Springer-Verlag, Heidelberg, Germany, 371-399.
Keller, M., Jacob,
D.J., Wofsy, S.C., and Harriss, R.C.: 1991,
`Effects of tropical deforestation on global and regional atmospheric
chemistry', Clim. Change 19(1-2): 139-158.
Keller, M., Kaplan,
W.A., and Wofsy, S.C.: 1986, `Emissions of N2O, CH4 and
CO2 from tropical forest soils', J. Geophys. Res. 91:
11,791-11,802.
Keller, M., Veldkamp,
E., Weltz A.M., and Reiners, W.: 1993,
`Effect of pasture age on soil trace-gas emissions from a deforested
area of Costa Rica', Nature 365: 244-246.
Klink, C.A., Macedo, R.H., and Mueller, C.C.: 1994, Cerrado: Processo de Ocupação e Implicações para a Conservação e Utilização da sua Diversidade Biológica, World Wide Fund for Nature (WWF)-Brasil, Brasilia.
Kuhlbusch, T.A.J., and
Crutzen, P.J.: 1995, `Toward a global estimate of black carbon in residues of
vegetation fires representing a sink of atmospheric CO2 and a source
of O2', Global Biogeochem. Cycles, in press.
Lugo, A.E., Sanchez,
M.M., and Brown, S.: 1986, `Land use and organic carbon content of some
subtropical soils', Plant and Soil 96: 185-196.
Luizão, F., Matson,
P., Livingston, G., Luizão, R., and Vitousek, P.: 1989, `Nitrous oxide flux
following tropical land clearing', Global Biogeochem. Cycles 3:
281-285.
Makundi, W.R.,
Sathaye, J.A., and Masera, O.: 1992, Summary,
Carbon Emissions and Sequestration in Forests: Case Studies from Developing
Countries, Volume 1, LBL-32758, UC-402, Climate Change Division, Environmental
Protection Agency (EPA), Washington, DC and Energy and Environment Division,
Lawrence Berkeley Laboratory (LBL), University of California (UC), Berkeley,
CA.
Martius, C.: 1989, Untersuchungen
zur Ökologie des Holzabbaus durch Termiten (Isoptera) in zentralamazonischen
Überschwemmunsgswäldem (Várzea), AFRAA-Verlag, Frankfurt, Germany.
Martius, C.,
Fearnside, P.M., Bandeira, A.G. and Wassmann, R.: 1996, `Deforestation and
methane release from termites in Amazonia', Chemosphere, 33,
517-536.
Martius, C., Wassmann,
R., Thein, U., Bandeira, A., Rennenberg, H., Junk, W., and Seiler, W.: 1993,
`Methane emissions from wood-feeding termites in Amazonia', Chemosphere 26(1-4):
623-632.
Mooney, H.A.,
Vitousek, P.M., and Matson, P.A.: 1987, `Exchange of materials between
terrestrial ecosystems and the atmosphere', Science 238: 926-932.
Muzio, L.J. and
Kramlich, J.C.: 1988, `An artifact in the measurement of N2O from
combustion sources', Geophys. Res. Letters 15: 1369-1372.
Nepstad, D.C., de
Carvalho, C.R., Davidson, E.A., Jipp, P.H.
Lefebvre, P.A., Negreiros, G.H. da Silva, E.D., Stone, T.A. Trumbore,
S.E., and Vieira, S.: 1994, `The role of deep roots in the hydrological and
carbon cycles of Amazonian forests and pastures', Nature 372:
666-669.
Nye, P.H. and
Greenland, D.J.: 1960, The Soil Under Shifting Cultivation, Technical
Comunication No. 51, Commonwealth Agricultural Bureaux of Soils, Harpenden, UK.
Post, W.M., Emanuel,
W.R., Zinke, P.J., and Strangenberger, A.G.: 1982, `Soil carbon pools and world
life zones', Nature 298: 156-159.
Rasmussen, R.A. and
Khalil, M.A.K.: 1983, `Global production of methane by termites', Nature
301: 700-702.
Rasmussen, R.A. and Khalil, M.A.K.: 1988, `Isoprene over the Amazon Basin', J. Geophys. Res. 93: 1417-1421.
Revilla Cardenas, J.D., Kahn, F.L, and Guillaumet, J.L.: 1982, `Estimativa da Fitomassa do Reservatório da UHE de Tucuruí', in Brazil, Presidência da República, Ministério das Minas e Energia, Centrais Elétricas do Norte S.A. (ELETRONORTE) and Brazil, Secretaria do Planejamento, Conselho Nacional de Desenvolvimento Científico e Tecnológico, Instituto Nacional de Pesquisas da Amazônia (SEPLAN‑CNPq‑INPA), Projeto Tucuruí, Relatório Semestral Jan.‑Jun. 1982, Vol. 2: Limnologia, Macrófitas, Fitomassa, Degradação de Fitomassa, Doenças Endêmicas, Solos, INPA, Manaus, Amazonas, 1-11.
Richey, J.E., Brock,
J.T., Naiman, R.J., Wissmar, R.C., and Stallard, R.R.: 1980, `Organic carbon:
Oxidation and transport in the Amazon River', Science 207:
1348-1351.
Saldarriaga, J.G.,
West, D.C., and Tharp, M.L.: 1986, Forest Succession in the Upper Rio Negro
of Colombia and Venezuela, Oak Ridge National Laboratory, Environmental
Sciences Publication No. 2694, ORNL/TM-9712, National Technical Information
Service, Springfield, VA.
Seiler, W. and
Crutzen, P.J.: 1980, `Estimates of gross and net fluxes of carbon between the
biosphere and the atmosphere from biomass burning', Clim. Change 2:
207-247.
Seiler, W., Conrad,
R., and Scharffe, D.: 1984, `Field studies of methane emission from termite
nests into the atmosphere and measurements of methane uptake by tropical soils',
J. Atmos. Chem. 1: 171-186.
Shine, K.P., Derwent,
R.G., Wuebbles, D.J., and Morcrette, J-J.: 1990, 'Radiative forcing of
climate', in Houghton, J.T., Jenkins, G.J., and Ephraums, J.J. (eds.) Climate
Change: The IPCC Scientific Assessment, Cambridge University Press,
Cambridge, UK, 41-68.
Skole, D. and Tucker,
C.: 1993. `Tropical deforestation and habitat fragmentation in the Amazon:
Satellite data from 1978 to 1988', Science 260: 1905-1910.
Shukla, J., Nobre, C.,
and Sellers, P.: 1990, `Amazon deforestation and climate change', Science
247: 1322-1325.
Thompson, A.M. and
Cicerone, R.J.: 1986, `Possible perturbations to atmospheric CO, CH4,
and OH', J. Geophys. Res. 91(D10): 10,853-10,864.
Trumbore, S.E.,
Davidson, E.A., de Camargo, P.B., Nepstad, D.C., and Martinelli, L.A.: 1995.
`Belowground cycling of carbon in forests and pastures of Eastern Amazonia', Global
Biogeochem. Cycles 9: 515-528.
Uhl, C. and
Saldarriaga, J.: nd, `The disappearance of wood mass following slash and burn
agriculture in the Venezuelan Amazon', (manuscript).
United Nations
Educational Scientific and Cultural Programme (UNESCO)/United Nations
Environment Programme (UNEP)/Food and Agricultural Organization of the United
Nations (FAO): 1978, Tropical Forest
Ecosystems: A State of Knowledge Report, UNESCO, Paris.
Veldkamp, E.: 1993, Soil
Organic Carbon Dynamics in Pastures Established after Deforestation in the
Humid Tropics of Costa Rica, Ph.D. dissertation, Agricultural University
Wageningen, Wageningen, The Netherlands, 117 pp.
Vermeer, D.E.: 1970,
`Population pressure and crop rotational changes among the Tiv of Nigeria', Annals
of the Association of American Geographers 60: 299-314.
Ward, D.E.: 1986,
`Field scale measurements of emission from open fires', technical paper
presented at the Defense Nuclear Agency Global Effects Review, Defense Nuclear
Agency, Washington, DC.
Ward, D.E. and Hardy,
C.C.: 1984, `Advances in the characterization and control of emissions from
prescribed fires', paper presented at the 77th annual meeting of the Air
Pollution Control Association, San Francisco, CA.
Wood, T.G., Johnson,
R.A., and Ohiagu, C.: 1977, `Populations of termites (Isoptera) in natural and
agricultural ecosystems in southern Guinea savanna near Mokwa, Nigeria', in
Malaisse, F., (ed.) Structure,
Fonctionnement et Amenagement d'Ecosystemes Tropicaux (Geo-Eco-Trop Vol. 1, No. 2), Faculté des
Sciences, Université Nationale du Zaïre, Lubumbashi, Zaïre, 139-148.
Zimmerman, P.R.,
Greenberg, J.P., and Darlington, J.P.E.C.: 1984, `Termites and atmospheric gas production', Science
224: 86.
Zimmerman, P.R.,
Greenberg, J.P., Wandiga, S.O., and Crutzen, P.J.: 1982, `Termites: A
potentially large source of atmospheric methane, carbon dioxide, and molecular
hydrogen', Science 218: 563-565.
FIGURE LEGENDS
Fig. 1.Brazil's Legal Amazon region, with
locations mentioned in the text.
Fig. 2.Forest and nonforest in the Brazilian
Legal Amazon (Source: Fearnside and Ferraz, 1995).
Fig. 3.Carbon transformations through a typical
10-year sequence of clearing, burning and reburning (updated from: Fearnside,
1991).
Fig. 4.Partioning of carbon between type of
release and emitted gas (Low trace gas scenario) (updated from: Fearnside,
1991).
TABLE I: EXTENT OF DEFORESTATION IN THE BRAZILIAN
LEGAL AMAZON(a) |
|
|
|
|
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Original |
Deforested
area |
|
|
|
Deforested
area |
|
|
|
||
|
Political |
forest |
(km2
X 103) |
|
|
|
(%
of original forest area) |
|
|
|||
|
unit |
area |
|
|
|
|
|
|
|
|
|
|
|
|
(km2
X 103) |
Jan.
1978 |
Apr.
1988 |
Aug.
1989 |
Aug.
1990 |
Aug.
1991 |
Jan.
1978 |
Apr.
1988 |
Aug.
1989 |
Aug.
1990 |
Aug.
1991 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Acre |
152 |
2.6 |
8.9 |
9.8 |
10.3 |
10.7 |
1.7 |
5.8 |
6.4 |
6.8 |
7.0 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Amapá |
115 |
0.2 |
0.9 |
1.0 |
1.3 |
1.7 |
0.2 |
0.8 |
0.9 |
1.1 |
1.5 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Amazonas |
1,481 |
2.3 |
18.0 |
19.3 |
19.8 |
20.8 |
0.2 |
1.2 |
1.3 |
1.3 |
1.4 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Maranhão |
143 |
65.9(b) |
90.8(b) |
92.3(b) |
93.4(b) |
94.1(b) |
46.1 |
63.5 |
64.5 |
65.3 |
65.8 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Mato
Grosso |
528 |
26.5 |
71.5 |
79.6 |
83.6 |
86.5 |
5.0 |
13.5 |
15.1 |
15.8 |
16.4 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Pará |
1,139 |
61.7(b) |
129.6(b) |
137.3(b) |
142.2(b) |
146.0(b) |
5.4 |
11.4 |
12.1 |
12.5 |
12.8 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Rondônia |
215 |
6.3 |
29.6 |
31.4 |
33.1 |
34.2 |
2.9 |
13.8 |
14.6 |
15.4 |
15.9 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Roraima |
164 |
0.2 |
2.8 |
3.6 |
3.8 |
4.2 |
0.1 |
1.7 |
2.2 |
2.3 |
2.6 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Tocantins |
59 |
4.2 |
21.6 |
22.3 |
22.9 |
23.4 |
7.1 |
36.7 |
37.9 |
38.9 |
39.7 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Legal |
3,996 |
169.9 |
373.9 |
396.6 |
410.4 |
421.6 |
4.3 |
9.4 |
9.9 |
10.3 |
10.5 |
|
Amazon |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
FOREST
FLOODED BY HYDROELECTRIC DAMS |
|
|
|
|
|
|
|||
|
Legal |
|
|
|
|
|
|
|
|
|
|
|
|
Amazon |
|
0.1 |
4.4 |
5.5 |
5.5 |
5.5 |
0.0 |
0.1 |
0.1 |
0.1 |
0.1 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
DEFORESTATION
FROM ALL SOURCES |
|
|
|
|
|
|
|||
|
Legal |
|
|
|
|
|
|
|
|
|
|
|
|
Amazon |
|
169.9 |
378.3 |
402.1 |
416.0 |
427.1 |
4.3 |
9.5 |
10.1 |
10.4 |
10.7 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(a)
Sources: Fearnside, 1993a,b |
|
|
|
|
|
|
|
|
|||
|
(b)
Maranhão values include 57.8 X 103 km2, and Pará values
include 39.8 X 103 km2, of |
|
|
|||||||||
|
"old" (approximately pre‑1970)
deforestation now largely under secondary forest. |
|
|
|||||||||
|
TABLE II: RATE OF DEFORESTATION IN THE BRAZILIAN
LEGAL AMAZON(a) |
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Political |
Deforestation
rate (km2 X 103/year) |
|
|||
|
unit |
|
|
|
|
|
|
|
1978‑1988 |
1988‑1989 |
1989-1990 |
1990‑1991 |
|
|
|
|
|
|
|
|
|
|
DEFORESTATION
EXCLUSIVE OF HYDROELECTRIC DAMS |
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Acre |
0.6 |
0.6 |
0.6 |
0.4 |
|
|
|
|
|
|
|
|
|
Amapá |
0.1 |
0.2 |
0.3 |
0.4 |
|
|
|
|
|
|
|
|
|
Amazonas |
1.6 |
1.3 |
0.5 |
1.0 |
|
|
|
|
|
|
|
|
|
Maranhão |
2.5 |
1.4 |
1.1 |
0.7 |
|
|
|
|
|
|
|
|
|
Mato
Grosso |
4.5 |
6.0 |
4.0 |
2.8 |
|
|
|
|
|
|
|
|
|
Pará |
6.8 |
5.8 |
4.9 |
3.8 |
|
|
|
|
|
|
|
|
|
Rondônia |
2.1 |
1.4 |
1.7 |
1.1 |
|
|
|
|
|
|
|
|
|
Roraima |
0.2 |
0.7 |
0.2 |
0.4 |
|
|
|
|
|
|
|
|
|
Tocantins |
1.6 |
0.7 |
0.6 |
0.4 |
|
|
|
|
|
|
|
|
|
Legal |
20.0 |
18.1 |
13.8 |
11.1 |
|
|
Amazon |
|
|
|
|
|
|
|
FOREST
FLOODED BY HYDROELECTRIC DAMS |
|
|||
|
Legal |
|
|
|
|
|
|
Amazon |
0.4 |
1.1 |
0.0 |
0.0 |
|
|
|
|
|
|
|
|
|
|
DEFORESTATION
FROM ALL SOURCES |
|
|
||
|
Legal |
|
|
|
|
|
|
Amazon |
20.4 |
19.2 |
13.8 |
11.1 |
|
|
|
|
|
|
|
|
|
(a)
Sources: Fearnside, 1993a,b |
|
|
|
||
TABLE
III: VEGETATION TYPES IN THE BRAZILIAN
LEGAL AMAZON(a)
‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑----
Cate‑ Code
Group
Subgroup Class
gory
‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑----
Dense Da‑0 Ombrophilous forest Dense forest Alluvial Amazonian
Forest Db‑0 Ombrophilous forest Dense forest Lowland Amazonian
Dm‑0 Ombrophilous forest Dense forest Montane Amazonian
Ds‑0 Ombrophilous forest Dense forest Submontane Amazonian
‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑----
‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑----
Non‑ Aa‑0 Ombrophilous forest Open Alluvial
dense Ab‑0 Ombrophilous forest Open Lowland
forest As‑0 Ombrophilous forest Open Submontane
Cs‑0 Seasonal forest Deciduous Submontane
Fa‑0 Seasonal forest Semideciduous Alluvial
Fs‑0 Seasonal forest Semideciduous Submontane
La‑0 Woody oligotrophic vegetation of swampy
and sandy areas Open arboreous
Ld‑0 Woody oligotrophic vegetation of swampy
and sandy areas Dense arboreous
Lg‑0 Woody oligotrophic vegetation of swampy
and sandy areas Grassy‑woody
LO‑0 Areas of ecological tension and
contact Woody
oligotrophic vegetation of swampy
and sandy
areas‑‑ombrophilous forest
ON‑0 Areas of ecological tension and
contact Ombrophilous
forest‑‑seasonal forest
Pf‑0 Areas of pioneer formations Fluvio‑marine
influence
SM‑0 Areas of ecological tension and
contact Savanna‑‑dense
ombrophilous forest
SN‑0 Areas of ecological tension and
contact Savanna‑‑seasonal
forest
SO‑0 Areas of ecological tension and
contact Savanna‑‑ombrophilous
forest
‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑----
‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑----
Non‑ Pa‑0 Areas of pioneer formations Fluvial influence
forest rm‑0 Ecological refugium High altitude Montane
Sa‑0
Savanna Cerrado Open arboreous
Sd‑0 Savanna Cerrado Dense arboreous
Sg‑0 Savanna Cerrado Grassy‑woody
Sp‑0
Savanna
Cerrado
Parkland
ST‑0 Areas of ecological tension and
contact Savanna‑‑steppe‑like
savanna
Td‑3 Steppe‑like savanna Roraima grasslands Dense arboreous
Tp‑3 Steppe‑like savanna Roraima grasslands Parkland
‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑----
(a)
Source: Brazil, IBGE and IBDF, 1988.
|
TABLE IV: AREA OF NATURAL VEGETATION PRESENT IN THE
BRAZILIAN LEGAL AMAZON(a) |
|
|
|||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cate‑ |
Code |
Acre |
Amapá |
Amazonas |
Maranhão |
Mato |
Pará |
Rondônia |
Roraima |
Tocantins/ |
Total |
|
gory |
|
|
|
|
|
Grosso |
|
|
|
Goiás |
present |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Dense |
Da‑0 |
|
9,011 |
164,876 |
|
|
76,570 |
2,704 |
3,326 |
2,610 |
259,097 |
|
forest |
Db‑0 |
16,408 |
2,184 |
615,203 |
22,586 |
|
164,091 |
2,066 |
10,248 |
|
832,786 |
|
|
Dm‑0 |
|
113 |
10,181 |
|
|
3,418 |
|
20,661 |
|
34,373 |
|
|
Ds‑0 |
518 |
99,220 |
178,103 |
1,988 |
23,154 |
413,345 |
14,607 |
83,692 |
3,055 |
817,682 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Subtotal |
16,926 |
110,528 |
968,363 |
24,574 |
23,154 |
657,424 |
19,377 |
117,927 |
5,665 |
1,943,938 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Non‑ |
Aa‑0 |
10,591 |
|
65,748 |
|
|
805 |
2,273 |
|
|
79,417 |
|
dense |
Ab‑0 |
114,380 |
|
211,052 |
|
|
|
41,064 |
|
|
366,496 |
|
forest |
As‑0 |
|
|
37,555 |
|
124,620 |
286,271 |
77,794 |
8,430 |
1,216 |
535,886 |
|
|
Cs‑0 |
|
|
|
3,666 |
736 |
5,386 |
|
|
115 |
9,903 |
|
|
Fa‑0 |
|
|
|
|
3,554 |
|
|
|
|
3,554 |
|
|
Fs‑0 |
|
|
|
|
24,317 |
|
7,718 |
1,041 |
1,328 |
34,404 |
|
|
La‑0 |
|
|
14,979 |
|
|
|
|
970 |
|
15,949 |
|
|
Ld‑0 |
|
|
37,405 |
|
|
|
|
10,967 |
|
48,372 |
|
|
Lg‑0 |
|
|
9,663 |
|
|
|
|
9,767 |
|
19,430 |
|
|
LO‑0 |
|
|
172,607 |
|
|
|
|
30,184 |
|
202,791 |
|
|
ON‑0 |
|
|
|
|
168,069 |
2,991 |
4,801 |
3,045 |
|
178,906 |
|
|
Pf‑0 |
|
1,823 |
|
2,089 |
|
3,894 |
|
|
|
7,806 |
|
|
SM‑0 |
|
|
|
384 |
|
|
|
|
|
384 |
|
|
SN‑0 |
|
|
1,082 |
6,570 |
142,778 |
27,812 |
4,781 |
904 |
14,465 |
198,392 |
|
|
SO‑0 |
|
4,226 |
27,350 |
|
22,124 |
59,734 |
21,932 |
4,286 |
6,551 |
146,203 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Subtotal |
124,971 |
6,049 |
577,441 |
12,709 |
486,198 |
386,893 |
160,363 |
69,594 |
23,675 |
1,847,893 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Subtotal |
141,897 |
116,577 |
1,545,804 |
37,283 |
509,352 |
1,044,317 |
179,740 |
187,521 |
29,340 |
3,791,831 |
|
|
all
forests |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Non‑ |
Pa‑0 |
|
15,157 |
12,778 |
2,517 |
14,738 |
27,162 |
8,690 |
|
|
81,042 |
|
forest |
rm‑0 |
|
|
|
|
|
|
|
390 |
|
390 |
|
|
Sa‑0 |
|
|
1,531 |
55,758 |
167,534 |
5,686 |
11,028 |
|
102,445 |
343,982 |
|
|
Sd‑0 |
|
|
|
15,771 |
10,840 |
1,274 |
|
|
2,234 |
30,119 |
|
|
Sg‑0 |
|
|
|
|
10,490 |
5,057 |
|
15,481 |
7,113 |
38,141 |
|
|
Sp‑0 |
|
10,038 |
5,556 |
26,980 |
64,085 |
12,393 |
2,664 |
8,969 |
48,962 |
179,647 |
|
|
ST‑0 |
|
|
|
|
6,599 |
|
|
|
|
6,599 |
|
|
Td‑3 |
|
|
|
|
|
|
|
1,550 |
|
1,550 |
|
|
Tp‑3 |
|
|
|
|
|
|
|
10,671 |
|
10,671 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Subtotal |
0 |
25,195 |
19,865 |
101,026 |
274,286 |
51,572 |
22,382 |
37,061 |
160,754 |
692,141 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total |
141,897 |
141,772 |
1,565,669 |
138,309 |
783,638 |
1,095,889 |
202,122 |
224,582 |
190,094 |
4,483,972 |
|
(a)
Areas in km2 measured from 1:5,000,000 vegetation map (Brazil,
IBGE and IBDF, 1988). |
|
|
|||||||||
|
These areas do not reflect losses due to recent
deforestation. |
|
|
|
|
|||||||
|
TABLE V: FOREST BIOMASS PER HECTARE: MEANS BY
ECOREGION, VEGETATION TYPE AND STATE (t/ha)(a) |
|
|
|
|||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cate‑ |
Code |
Acre |
Amapá |
Amazonas |
Maranhão |
Mato |
Pará |
Rondônia |
Roraima |
Tocantins/ |
Mean
in |
Area‑ |
|
gory |
|
|
|
|
|
Grosso |
|
|
|
Goiás |
sampled |
weighted |
|
|
|
|
|
|
|
|
|
|
|
|
plots |
mean |
|
Dense |
Da‑0 |
|
471 |
513 |
|
|
412 |
310 |
419 |
500 |
498 |
478 |
|
forest |
Db‑0 |
438 |
583 |
495 |
458 |
|
585 |
546 |
417 |
|
551 |
510 |
|
|
Dm‑0 |
|
441 |
344 |
|
|
441 |
|
448 |
|
440 |
417 |
|
|
Ds‑0 |
391 |
613 |
478 |
464 |
402 |
518 |
464 |
429 |
111 |
430 |
506 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Dense |
437 |
601 |
493 |
459 |
402 |
522 |
452 |
431 |
290 |
519 |
502 |
|
|
forests |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Non‑ |
Aa‑0 |
425 |
|
435 |
|
|
536 |
431 |
|
|
430 |
434 |
|
dense |
Ab‑0 |
455 |
|
466 |
|
|
|
403 |
|
|
455 |
456 |
|
forest |
As‑0 |
|
|
507 |
|
426 |
361 |
378 |
376 |
376 |
374 |
389 |
|
|
Cs‑0 |
|
|
|
384 |
384 |
384 |
|
|
384 |
384 |
384 |
|
|
Fa‑0 |
|
|
|
|
370 |
|
|
|
|
370 |
370 |
|
|
Fs‑0 |
|
|
|
|
403 |
|
472 |
424 |
424 |
423 |
420 |
|
|
La‑0 |
|
|
465 |
|
|
|
|
465 |
|
|
435 |
|
|
Ld‑0 |
|
|
437 |
|
|
|
|
437 |
|
|
435 |
|
|
Lg‑0 |
|
|
437 |
|
|
|
|
437 |
|
|
435 |
|
|
LO‑0 |
|
|
500 |
|
|
|
|
437 |
|
499 |
490 |
|
|
ON‑0 |
|
|
|
|
383 |
401 |
547 |
409 |
|
401 |
389 |
|
|
Pf‑0 |
|
437 |
|
464 |
|
437 |
|
|
|
|
435 |
|
|
SM‑0 |
|
|
|
437 |
|
|
|
|
|
|
435 |
|
|
SN‑0 |
|
|
409 |
385 |
383 |
479 |
385 |
336 |
385 |
385 |
397 |
|
|
SO‑0 |
|
404 |
582 |
|
360 |
410 |
404 |
404 |
404 |
402 |
433 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Non‑dense |
|
453 |
414 |
478 |
399 |
394 |
379 |
399 |
425 |
392 |
435 |
423 |
|
forests |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
All |
|
451 |
591 |
488 |
438 |
394 |
469 |
404 |
429 |
372 |
500 |
464 |
|
forests |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||
|
(a)
Values in italics are for ecoregions where no sample exists: values are based on the mean in |
|
|
||||||||||
|
sampled plots for the same vegetation
type in other states. Source:
Fearnside, nd‑a. |
|
|
|
|||||||||
|
TABLE VI: APPROXIMATE BIOMASS CLEARED IN 1990 IN EACH
ECOREGION IN THE BRAZILIAN LEGAL AMAZON (103 t/year) |
|||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cate‑ |
Code |
Acre |
Amapá |
Amazonas |
Maranhão |
Mato |
Pará |
Rondônia |
Roraima |
Tocantins/ |
Total |
|
gory |
|
|
|
|
|
Grosso |
|
|
|
Goiás |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Dense |
Da‑0 |
|
944 |
2,924 |
|
|
15,013 |
733 |
125 |
2,600 |
22,339 |
|
forest |
Db‑0 |
2,846 |
293 |
10,492 |
29,196 |
|
44,014 |
1,106 |
384 |
|
88,331 |
|
|
Dm‑0 |
|
11 |
77 |
|
|
717 |
|
807 |
|
1,613 |
|
|
Ds‑0 |
80 |
13,993 |
2,981 |
2,978 |
7,391 |
99,957 |
6,400 |
3,015 |
689 |
137,484 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
subtotal |
2,926 |
15,242 |
16,474 |
32,175 |
7,391 |
159,702 |
8,238 |
4,331 |
3,288 |
249,767 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Non‑ |
Aa‑0 |
1,780 |
|
1,020 |
|
|
205 |
961 |
|
|
3,966 |
|
dense |
Ab‑0 |
20,448 |
|
3,472 |
|
|
|
16,183 |
|
|
40,103 |
|
forest |
As‑0 |
|
|
669 |
|
42,163 |
49,222 |
27,044 |
284 |
931 |
120,313 |
|
|
Cs‑0 |
|
|
|
4,551 |
225 |
984 |
|
|
90 |
5,850 |
|
|
Fa‑0 |
|
|
|
|
1,044 |
|
|
|
|
1,044 |
|
|
Fs‑0 |
|
|
|
|
7,786 |
|
3,576 |
40 |
777 |
12,178 |
|
|
La‑0 |
|
|
239 |
|
|
|
|
40 |
|
279 |
|
|
Ld‑0 |
|
|
577 |
|
|
|
|
411 |
|
989 |
|
|
Lg‑0 |
|
|
151 |
|
|
|
|
383 |
|
534 |
|
|
LO‑0 |
|
|
2,815 |
|
|
|
|
1,121 |
|
3,936 |
|
|
ON‑0 |
|
|
|
|
51,195 |
570 |
2,574 |
112 |
|
54,451 |
|
|
Pf‑0 |
|
28 |
|
3,129 |
|
810 |
|
|
|
3,967 |
|
|
SM‑0 |
|
|
|
543 |
|
|
|
|
|
543 |
|
|
SN‑0 |
|
|
16 |
8,173 |
42,641 |
6,337 |
1,805 |
27 |
11,348 |
70,347 |
|
|
SO‑0 |
|
319 |
569 |
|
6,334 |
11,647 |
7,506 |
155 |
5,395 |
31,926 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Subtotal |
22,228 |
347 |
9,528 |
16,396 |
151,387 |
69,778 |
59,648 |
2,573 |
18,541 |
350,426 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Subtotal |
25,155 |
15,589 |
26,002 |
48,571 |
158,779 |
229,480 |
67,886 |
6,904 |
21,829 |
600,193 |
|
|
all
forests |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Average |
Dense |
|
|
|
|
|
|
|
|
|
|
|
biomass/ |
forests |
437 |
601 |
494 |
459 |
402 |
522 |
453 |
431 |
288 |
500 |
|
ha |
|
|
|
|
|
|
|
|
|
|
|
|
cleared |
|
|
|
|
|
|
|
|
|
|
|
|
|
non‑dense |
453 |
407 |
478 |
399 |
394 |
379 |
399 |
425 |
391 |
397 |
|
|
forests |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
All |
451 |
595 |
488 |
437 |
394 |
468 |
405 |
429 |
371 |
434 |
|
|
forests |
|
|
|
|
|
|
|
|
|
|
|
TABLE VII: ANNUAL PROBABILITIES OF TRANSFER |
|||||||
|
|
|
|
|
|
|
|
|
|
Initial
state |
|
Subsequent
state |
|
|
|
|
|
|
|
|
Regener‑ |
Farm‑ |
Produc‑ |
Degraded |
Secondary |
Secondary |
|
|
|
ated |
land |
tive |
pasture |
forest |
forest |
|
|
|
Forest |
|
pasture |
|
from |
from |
|
|
|
|
|
|
|
farmland |
pasture |
|
|
|
|
|
|
|
|
|
|
Regener‑ |
|
|
|
|
|
|
|
|
ated |
|
0 |
0.347 |
0.653 |
0 |
0 |
0 |
|
Forest |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Farmland |
|
0 |
0.450 |
0.468 |
0 |
0.082 |
0 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Productive |
0 |
0 |
0.849 |
0.008 |
0 |
0.143 |
|
|
pasture |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Degraded |
|
0 |
0 |
0.007 |
0.926 |
0 |
0.067 |
|
pasture |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Secondary |
|
5.17 X 10-10 |
0.065 |
0.128 |
0 |
0.807 |
0 |
|
forest
from |
|
|
|
|
|
|
|
|
farmland |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Secondary |
|
|
|
|
|
|
|
|
forest
from |
7.16 X 10‑8 |
0.061 |
0.101 |
0 |
0 |
0.838 |
|
|
pasture |
|
|
|
|
|
|
|
|
TABLE VII: ANNUAL PROBABILITIES OF TRANSFER |
|||||||
|
|
|
|
|
|
|
|
|
|
Initial
state |
|
Subsequent
state |
|
|
|
|
|
|
|
|
Regener‑ |
Farm‑ |
Produc‑ |
Degraded |
Secondary |
Secondary |
|
|
|
ated |
land |
tive |
pasture |
forest |
forest |
|
|
|
Forest |
|
pasture |
|
from |
from |
|
|
|
|
|
|
|
farmland |
pasture |
|
|
|
|
|
|
|
|
|
|
Regener‑ |
|
|
|
|
|
|
|
|
ated |
|
0 |
0.347 |
0.653 |
0 |
0 |
0 |
|
Forest |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Farmland |
|
0 |
0.450 |
0.468 |
0 |
0.082 |
0 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Productive |
0 |
0 |
0.849 |
0.008 |
0 |
0.143 |
|
|
pasture |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Degraded |
|
0 |
0 |
0.007 |
0.926 |
0 |
0.067 |
|
pasture |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Secondary |
|
5.17 X 10-10 |
0.065 |
0.128 |
0 |
0.807 |
0 |
|
forest
from |
|
|
|
|
|
|
|
|
farmland |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Secondary |
|
|
|
|
|
|
|
|
forest
from |
7.16 X 10‑8 |
0.061 |
0.101 |
0 |
0 |
0.838 |
|
|
pasture |
|
|
|
|
|
|
|
|
TABLE VIII: REPLACEMENT VEGETATION WEIGHTED BIOMASS
CALCULATION AT EQUILIBRIUM |
||||
|
|
|
|
|
|
|
Category |
Equilib‑ |
Biomass |
Resid‑ |
Biomass |
|
|
rium |
(t/ha |
ence |
source |
|
|
propor‑ |
total) |
time |
|
|
|
tion |
|
(years) |
|
|
Forest |
0.000 |
464 |
0.0 |
(a) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Farmland |
0.040 |
0.7 |
0.9 |
(b) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Productive |
0.438 |
10.7 |
4.2 |
(c) |
|
Pasture |
|
|
|
|
|
|
|
|
|
|
|
Degraded |
0.052 |
8.0 |
9.1 |
(d) |
|
pasture |
|
|
|
|
|
|
|
|
|
|
|
Secondary |
0.020 |
35.6 |
3.2 |
(d) |
|
forest |
|
|
|
|
|
from
agriculture |
|
|
|
|
|
|
|
|
|
|
|
Secondary |
0.449 |
50.5 |
3.9 |
(d) |
|
forest |
|
|
|
|
|
from
pasture |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted
mean: |
|
28.5 |
|
|
|
|
|
|
|
|
(a)Secondary forest is assumed to be equivalent
to original forest from the standpoint of biomass after 100 years. Saldarriaga et al. (1986: 96)
calculated recovery in 144‑189 years in Venezuela. Original forest biomass from Fearnside (nd‑a).
(b) Guess: above‑ground biomass=0.5 t/ha;
root/shoot ratio=0.3.
(c) Fearnside et al., nd‑b, see
Fearnside, 1989b.
(d) Calculated from the residence time and
growth rate (Fearnside and Guimaraes, 1996).
|
TABLE IX: PARAMETERS FOR TRANSFORMATIONS OF CARBON
STOCKS |
|
||||||||
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
||
|
Parameter |
|
Value |
Units |
Source |
|
Comment |
|
||
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
||
|
Total
biomass |
407.0 |
t/ha
dry weight |
Fearnside,
nd‑b |
Weighted
mean for areas being cleared in 1990 (after logging). |
|
||||
|
Carbon
content of forest biomass |
0.50 |
fraction
of dry weight |
Measured
at Manaus by Fearnside et al., 1993 |
Many authors use a value of 0.45, but without
reference to experimental data for its origin. Brown and Lugo (1984) use a value of 0.50. |
|
||||
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
||
|
Above
ground fraction |
0.759 |
|
Calculated
from Fearnside, nd‑b |
Total
above‑ground biomass/total biomass of 1990 areas at time of clearing. |
|
||||
|
|
|
|
|
|
|
|
|
||
|
Burning
efficiency in initial burn |
|
0.332 |
fraction
of C released |
Fearnside
et al., 1993, nd‑a,b adjusted for logging by Fearnside, nd-b |
For
areas cleared after preparatory logging. |
|
|||
|
|
|
|
|
|
|
|
|||
|
Char
C fraction in initial burn |
0.019 |
|
Fearnside
et al., 1993, nd‑a,b |
Average
of two studies near Manaus, Amazonas and one in Altamira, Pará. |
|
||||
|
|
|
|
|
|
|
|
|
||
|
Fraction
of char on biomass following initial burn |
0.89 |
|
Fearnside
et al., nd‑a |
|
Near
Altamira, Pará. |
|
|||
|
|
|
|
|
|
|
|
|
||
|
Exposed
to soil char C transfer fraction during first interval |
0.3 |
|
Guess |
|
First
interval = 5 years. |
|
|||
|
|
|
|
|
|
|
|
|
||
|
Fraction
surviving decay in first interval |
0.400 |
|
Calculated
from Uhl and Saldarriaga, nd and Buschbacher, 1984(a) |
|
|
||||
|
|
|
|
|
||||||
|
Burning
efficiency in first reburn |
|
0.201 |
fraction
of C released |
Fearnside,
et al., nd‑d; Barbosa, 1994 |
Burns
of secondary forest and pasture in Apiaú, Roraima |
|
|||
|
|
|
|
|
|
|
|
|
||
|
Fraction
converted to char in first reburn |
0.010 |
|
Fearnside,
et al., nd‑d; Barbosa, 1994 |
Burns
in Apiaú, Roraima (NB: includes charcoal from secondary forest, but not
secondary forest biomass) |
|
||||
|
|
|
|
|
|
|
|
|
||
|
Char
C combustion fraction in first reburn |
|
0 |
|
Assumed
zero because char conversion value is net |
|
||||
|
|
|
|
|
|
|
|
|
||
|
Fraction
surviving decay in second interval |
0.543 |
|
Calculated
from Uhl and Saldarriaga, nd(a) |
Second
interval = 3 years |
|
||||
|
|
|
|
|
|
|
|
|
||
|
Burning
efficiency in second reburn |
|
0.201 |
fraction
of C released |
Assumed
equal to first reburn |
|
|
|||
|
|
|
|
|
|
|
||||
|
Fraction
of C converted to char in second reburn |
0.010 |
|
Assumed
equal to first reburn |
|
|
||||
|
|
|
|
|
|
|
|
|
||
|
Fraction
of char on biomass after first reburn |
0.89 |
|
Assumed
equal to initial burn |
|
|
||||
|
|
|
|
|
|
|
||||
|
Exposed
to soil char C transfer fraction during second interval |
0.3 |
|
Guess |
|
|
|
|||
|
|
|
|
|
|
|
|
|
||
|
Char
C combusted fraction in second reburn |
0 |
|
Assumed
zero because char conversion value is net |
||||||
|
|
|
|
|
||||||
|
Fraction
of char on biomass after second reburn |
0.89 |
|
Assumed
equal to initial burn |
|
|
||||
|
|
|
|
|
|
|
||||
|
Exposed
to soil char C transfer fraction during third interval |
0.3 |
|
Guess |
|
|
|
|||
|
|
|
|
|
|
|
|
|
||
|
Fraction
surviving decay in third interval |
0.767 |
|
Calculated
from Uhl and Saldarriaga, nd. |
Third
interval = 3 years |
|
||||
|
|
|
|
|
|
|
|
|||
|
Burning
efficiency in third reburn |
|
0.201 |
fraction
of wood C released |
Assumed
equal to first reburn |
|
|
|||
|
|
|
|
|
|
|
||||
|
Fraction
of C converted to char in third reburn |
0.010 |
|
Assumed
equal to first reburn |
|
|
||||
|
|
|
|
|
|
|
|
|
||
|
Char
C combustion fraction in third reburn |
|
0 |
|
Assumed
zero because char conversion value is net |
|
||||
|
|
|
|
|
|
|
|
|
||
|
Soil
C release from top 20 cm |
|
3.92 |
t/ha |
Fearnside,
1985, 1987 |
|
|
|||
|
|
|
|
|
|
|
|
|
||
|
Replacement
vegetation biomass |
28.5 |
t/ha |
Table
VIII. |
|
Weighted
average for equilibrium landscape |
|
|||
|
|
|
|
|
|
|
|
|
||
|
Carbon
content of replacement vegetation biomass |
0.45 |
fraction
of dry weight |
Based
on measurements at Altamira by Guimarães, 1993. |
|
|
|
|||
|
|
|
|
|
|
|
||||
|
(a)
See Fearnside, in press-b |
|
|
|
|
|
||||
|
TABLE X: PARAMETERS FOR
CARBON EMISSIONS |
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Scenario |
Component |
|
|
Transformation |
|
Value
(t C released in this form / t C present in component) |
t
gas released/t fuel burned |
Basis
and reference |
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Both
high and low trace gas scenarios |
Above-ground
biomass carbon |
Combustion
release |
|
0.4203 |
|
Calculated
from parameters in Table IX and Figure 3 |
||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Decay
release |
|
0.5561 |
|
Calculated
from parameters in Table IX and Figure 3 |
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Charcoal
carbon formation (initial+subsequent burns) |
0.0237 |
|
Calculated
from parameters in Table IX and Figure 3 |
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
Carbon
released through combustion |
|
Initial
burn |
|
0.7905 |
|
Calculated
from parameters in Table IX and Figure 3 |
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Reburns |
|
|
0.2095 |
|
Calculated
from parameters in Table IX and Figure 3 |
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Combustion
release of below-ground biomass |
0 |
|
Assumption |
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
Carbon
released through decay |
|
Decay
release through termites (above ground) |
0.0297 |
|
Martius
et al., nd (average for first
10 years for original forest biomass;
emission calculations done with a different proportion for each year) |
||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
Decay
release through other decay (above ground) |
0.9703 |
|
One
minus decay release through termites |
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
Decay
release of below-ground biomass |
|
1 |
|
Assumption |
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
Decay
release through termites (below ground) |
0 |
|
Assumption
(unrealistically low) |
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
Decay
release through other decay (below ground) |
1 |
|
Assumption
(unrealistically high) |
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
Low
trace gas scenario |
Carbon
released by combustion in initial burn |
|
CH4
carbon |
|
0.0078 |
0.005 |
Kaufman
et al., 1990 from Ward, 1986(a) |
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CO2
carbon |
|
0.8779 |
1.55 |
Kaufman
et al., 1990 from Ward, 1986(a) |
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CO
carbon |
|
|
0.1068 |
0.12 |
Kaufman
et al., 1990 from Ward, 1986(a) |
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Carbon
released by combustion in reburns |
|
CH4
carbon |
|
0.0109 |
0.007 |
Kaufman
et al., 1990 from Ward, 1986(a) |
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CO2
carbon |
|
0.7930 |
1.4 |
Kaufman
et al., 1990 from Ward, 1986(a) |
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CO
carbon |
|
|
0.1958 |
0.22 |
Kaufman
et al., 1990 from Ward, 1986(a) |
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Carbon
released through termites |
|
CH4
carbon |
|
0.0022 |
0.001 |
Martius
et al., 1993, nd |
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CO2
carbon |
|
0.9978 |
1.996 |
Assumed
all C not released as methane is CO2. |
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
High
trace gas scenario |
Carbon
released by combustion in initial burn |
|
CH4
carbon |
|
0.0093 |
0.006 |
Kaufman
et al., 1990 from Ward, 1986(a) |
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CO2
carbon |
|
0.8779 |
1.55 |
Kaufman
et al., 1990 from Ward, 1986(a) |
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CO
carbon |
|
|
0.1335 |
0.15 |
Kaufman et al., 1990 from Crutzen, et al., 1985(a) |
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Carbon
released by combustion in reburns |
|
CH4
carbon |
|
0.0171 |
0.011 |
Kaufman et al., 1990 from Greenberg et al., 1984(a) |
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CO2
carbon |
|
0.7930 |
1.4 |
Kaufman
et al., 1990 from Ward, 1986(a) |
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CO
carbon |
|
|
0.2492 |
0.28 |
Kaufman
et al., 1990 from Greenberg et al., 1984 and Ward, 1986(a) |
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Carbon
released through termites |
|
CH4
carbon |
|
0.0079 |
0.005 |
Goreau
and de Mello, 1987 |
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CO2
carbon |
|
0.9978 |
|
Assumed
all C not released as methane is CO2 |
|
|||||
(a)
Calculated from data presented by Kaufman et al. (1990: 382) as
emission/kg fuel.
Carbon content of the experimental fuel is
assumed to be 48.2%, this value
being chosen such that all combusted carbon
is accounted for, either as CO2, CH4, CO,
NMHC or particulate graphitic carbon.
|
TABLE XI:
PARAMETERS FOR OTHER SOURCES OF GREENHOUSE GASES FROM LAND‑USE
CHANGE |
|
|
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
Factor |
|
|
Units |
Value |
Reference |
|
|
|
Note |
|
|
|
|
|
|
|
|
|
|
|
|
Soil
carbon from top 20 cm |
t
C/ha |
3.92 |
Fearnside,
1985 |
|
|
(a) |
|||
|
|
|
|
|
|
|
|
|
|
|
|
Cerrado biomass carbon |
t
C/ha |
17.27 |
Fearnside,
nd‑a |
|
|
(b) |
|||
|
|
|
|
|
|
|
|
|
|
|
|
Cattle
CH4 |
|
|
kg
CH4/head/year |
55 |
Ahuja, 1990, based on Crutzen et al., 1986 |
||||
|
|
|
|
|
|
|
|
|
|
|
|
Cattle
stocking rate |
|
head/ha |
0.3 |
Fearnside,
1979 |
|
|
(c) |
||
|
|
|
|
|
|
|
|
|
|
|
|
Pasture
soil N2O |
|
kg N2O/ha/year |
3.8 |
Luizão
et al., 1989 |
|
|
(d) |
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(a) For
conversion to pasture at Paragominas, based on Falesi (1976: 31 and 42) for
carbon contents |
|||||||||
|
and Hecht (1981: 95) for soil densities. |
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
(b) Based
on conversion to pasture (total biomass 10.7 t/ha) of cerrado with
average total biomass |
|||||||||
|
of 45 t/ha. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(c)
Feeding capacity after 3 years. |
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
(d)
Full annual cycle under pasture and forest at Manaus. |
|
|
|
|
|||||
|
TABLE XII: TRACE GAS PARAMETERS |
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Factor |
|
|
Gases |
|
Units |
Value |
Source |
|
|
|
|
|
|
|
|
|||
|
Intact
forest soil sink |
CH4 |
|
t
C/ha/yr |
‑0.0004 |
Keller
et al., 1986 |
|||
|
|
|
|
|
|
|
|
|
|
|
Burning
release |
|
N2O(a)(b) |
t
gas/t CO2 emitted from burn
|
0.0002 |
Cofer
et al., 1988 cited by Kaufman et al., 1990 |
|||
|
|
|
|
|
|
|
|
|
|
|
Burning
release |
|
N2O(c)(d) |
t gas/t C burned |
0.0017 |
Calculated
by Keller et al., 1991: 146 from Andreae et al., 1988 |
|||
|
|
|
|
|
|
|
|
|
|
|
Burning
release |
|
NOx(e) |
|
t gas/t C burned |
0.0079 |
Keller
et al., 1991: 146 |
||
|
|
|
|
|
|
|
|
|
|
|
Intact
forest release |
NOx(e) |
|
t
gas/ha/yr |
0.0131 |
Kaplan et al., 1988; see Keller et al., 1991 |
|||
|
Flaming
burn release |
Total
particulates |
|
t/t
CH4 gas from burn |
3.33 |
Calculated
by Kaufman et al., 1990: 380 from |
|||
|
|
|
|
|
|
|
Ward
and Hardy, 1984 and Ward, 1986 |
||
|
|
|
|
|
|
|
|
|
|
|
Smoldering
burn release |
Total
particulates |
|
t/t
CH4 gas from burn |
1.67 |
Calculated
by Kaufman et al., 1990: 380 from Ward and Hardy, 1984 and Ward, 1986 |
|||
|
|
|
|
|
|
|
|
|
|
|
Flaming
burn release |
NMHC(b) |
|
t/t
CH4 gas from burn |
0.67 |
Derived
using factor of 0.2 t NMHC/t particulates calculated by Kaufman et al.,
1990: 380 |
|||
|
|
|
|
|
|
|
|
|
|
|
Smoldering
burn release |
NMHC(b) |
|
t/t
CH4 gas from burn |
0.50 |
Derived
using factor of 0.3 t CH4/t particulates calculated by Kaufman et
al., 1990: 380 |
|||
|
|
|
|
|
|
|
|
|
|
|
Mixed
burn release |
|
NMHC(d)(f) |
t/t
C burned |
0.0131 |
Keller
et al., 1991: 146 from measurements of Andreae et al., 1988 |
|||
|
|
|
|
|
|
|
|
|
|
|
Intact
forest release |
NMHC |
|
t
gas/ha/yr |
0.12 |
Rasmussen
and Khalil, 1988: 1420 |
|||
|
(a)
Intact forest release accounted for in pasture soil calculation. |
|
|||||||
|
|
|
|
|
|
|
|
|
|
|
(b)
Used in low trace gas scenario. |
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
(c)
results in 0.109 t gas/ha burned, or three times the 0.041 t gas/t C burned
obtained using the parameter relating N2O to CO2. |
||||||||
|
|
|
|
|
|
|
|
|
|
|
(d)
Used in high trace gas scenario. |
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
(e)
NOx weight given in NO2 basis (following Shine et al.,
1990: 61). |
|
|||||||
|
|
|
|
|
|
|
|
|
|
|
(f) NMHC
emission corresponds to 0.85 t gas/ha burned, much higher than values derived
from methane, which are (for high and low trace gas scenarios, respectively):
0.36 and 0.43 t NMHC/ha burned for flaming combustion and 0.10 and 0.16 t
NMHC/ha burned for smoldering combustion. |
|
TABLE XIII: SOIL CARBON
PARAMETERS AND CALCULATIONS |
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Parameters |
|
|
|
|
|
Units |
Value |
|
Source |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
Soil
density in forest |
|
|
|
g/cm3 |
0.56
|
|
Hecht,
1981: 95 |
|
|
|||
|
|
Carbon
in forest soil |
|
|
|
% by
wt |
0.91
|
|
Falesi,
1976: 31 and 42 |
|
||||
|
|
Carbon
in pasture soil |
|
|
|
% by
wt |
0.56
|
|
Falesi,
1976: 31 and 42 |
|
||||
|
|
Top
20 cm C as fraction of 1 m C |
|
|
% by
wt |
42 |
|
Fearnside,
1987 |
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Calculated
values |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
Top 20 cm of soil: |
|
|
|
|
|
|
|
|
|
|
|
||
|
|
Soil
dry weight |
|
|
|
|
t/ha |
1,120 |
|
|
|
|
|
|
|
|
Carbon
in forest soil |
|
|
|
t/ha |
10.19 |
|
|
|
|
|
||
|
|
Carbon
in pasture soil compacted from top 20 cm of forest soil |
|
|
|
|
|
|
|
|
||||
|
|
Release
from top 20 cm |
|
|
|
t/ha |
3.92
|
|
|
|
|
|
||
|
|
Release
fraction of pre‑conversion soil C |
|
% by
wt |
38 |
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Top meter of soil: |
|
|
|
|
|
|
|
|
|
|
|
||
|
|
Soil
dry weight |
|
|
|
|
t/ha |
5,600
|
|
|
|
|
|
|
|
|
Carbon
in forest soil |
|
|
|
t/ha |
24.27
|
|
|
|
|
|
||
|
|
Carbon
in pasture soil |
|
|
|
t/ha |
14.93
|
|
|
|
|
|
||
|
|
Release
from top meter |
|
|
|
t/ha |
9.33
|
|
|
|
|
|
||
|
|
Release
fraction of pre‑conversion soil C |
|
% by
wt |
38 |
|
|
|
|
|
||||
|
TABLE XIV: NET COMMITTED GREENHOUSE GAS EMISSIONS BY SOURCE FOR 1990
CLEARING IN THE LEGAL AMAZON: LOW
TRACE GAS SCENARIO |
||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
Source |
|
|
|
Area |
Emissions (106 t of gas) |
|||||
|
|
|
|
|
affected |
|
|
|
|
|
|
|
|
|
|
|
(103
km2) |
|
|
|
|
|
|
|
|
|
|
|
|
CO2 |
CH4 |
CO |
N2O |
NOx |
NMHC |
|
FOREST |
|
|
|
|
|
|
|
|
|
|
|
|
Initial
burn |
|
13.8 |
228 |
0.74 |
17.66 |
0.05 |
0.56 |
0.49 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Reburns |
|
|
13.8 |
55 |
0.27 |
8.58 |
0.01 |
0.15 |
0.14 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Termites
above‑ground decay |
13.8 |
13 |
0.010 |
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Other
above‑ground decay |
13.8 |
422 |
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Below‑ground
decay |
|
13.8 |
249 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cattle(a) |
|
6.1 |
|
0.010 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Pasture
soil(a) |
|
6.1 |
|
|
|
0.002 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Loss
of intact forest |
7.3 |
|
0.0003 |
|
|
‑0.01 |
‑0.09 |
||
|
|
sources
and sinks(a) |
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Soil
carbon (top 20 cm) |
13.8 |
20 |
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Regrowth |
|
|
13.8 |
‑65 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Hydroelectric(a) |
|
0.0 |
|
0.00 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Forest
subtotal |
|
921 |
1.03 |
26.25 |
0.06 |
0.70 |
0.54 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CERRADO |
|
|
|
|
|
|
|
|
|
|
|
|
Initial
burn |
|
5.0 |
17 |
0.06 |
1.35 |
0.003 |
0.04 |
0.04 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Reburns |
|
|
5.0 |
2 |
0.01 |
0.26 |
0.0003 |
0.005 |
0.004 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Termites
above‑ground decay |
5.0 |
0.1 |
0.0001 |
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Other
above‑ground decay |
5.0 |
4 |
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Below‑ground
decay |
|
5.0 |
15 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cattle(a) |
|
5.0 |
|
0.008 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Pasture
soil(a) |
|
5.0 |
|
|
|
0.002 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Loss
of intact cerrado |
5.0 |
|
0.0002 |
|
|
‑0.0004 |
‑0.004 |
||
|
|
sources
and sinks(a)(b) |
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Soil
carbon (top 20 cm) |
5.0 |
7 |
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Regrowth |
|
|
5.0 |
‑9 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cerrado subtotal |
|
36 |
0.07 |
1.62 |
0.01 |
0.05 |
0.04 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
TOTAL
FOR LEGAL AMAZON |
|
|
958 |
1.10 |
27.86 |
0.06 |
0.74 |
0.58 |
||
|
|
|
|
|
|
|
|
|
|
|
|
|
(a)
Recurring effects (cattle methane, forest soil methane sink, pasture soil N20,
hydroelectric methane) summed for 100‑year period for consistency with
IPCC 100‑year horizon calculation. |
||||||||||
|
|
||||||||||
|
(b)
Intact cerrado source for NOx and NMHC derived from the
forest per‑hectare emission assuming emission is proportional to the tree
leaf dry weight biomass in each ecosystem.
Cerrado tree leaf biomass (dry season) = 0.756 t/ha (dos
Santos, 1989: 194). Forest (at
Tucuruí, Pará) = 12.94 t/ha (Revilla Cardenas et al., 1982: 6). |
||||||||||
|
TABLE XV: NET COMMITTED GREENHOUSE GAS EMISSIONS BY SOURCE FOR 1990
CLEARING IN THE LEGAL AMAZON: HIGH
TRACE GAS SCENARIO |
||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
Source |
|
|
|
Area |
Emissions (106 t of gas) |
|
|
|||
|
|
|
|
|
affected |
|
|
|
|
|
|
|
|
|
|
|
(103
km2) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CO2 |
CH4 |
CO |
N2O |
NOx |
NMHC |
|
FOREST |
|
|
|
|
|
|
|
|
|
|
|
|
Initial
burn |
|
13.8 |
228 |
0.88 |
22.08 |
0.12 |
0.56 |
0.93 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Reburns |
|
|
13.8 |
55 |
0.43 |
10.93 |
0.03 |
0.15 |
0.25 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Termites
above‑ground decay |
13.8 |
13 |
0.01 |
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Other
above‑ground decay |
13.8 |
422 |
|
|
|
|
|
||
|
|
Below‑ground
decay |
|
13.8 |
249 |
|
|
|
|
|
|
|
|
Cattle(a) |
|
6.1 |
|
0.01 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Pasture
soil(a) |
|
6.1 |
|
|
|
0.002 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Loss
of intact |
|
7.3 |
|
0.0003 |
|
|
‑0.01 |
‑0.09 |
|
|
|
forest
sources(a) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Soil
C stock |
|
13.8 |
20 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Regrowth |
|
|
13.8 |
‑65 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Hydroelectric(a) |
|
0.0 |
|
0.00 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Forest
subtotal |
|
921 |
1.33 |
33.00 |
0.15 |
0.70 |
1.08 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CERRADO |
|
|
|
|
|
|
|
|
|
|
|
|
Initial
burn |
|
5.0 |
17 |
0.07 |
1.69 |
0.009 |
0.043 |
0.07 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Reburns |
|
|
5.0 |
2 |
0.01 |
0.34 |
0.001 |
0.005 |
0.01 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Termites
above‑ground decay |
5.0 |
0.1 |
0.0001 |
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Other
above‑ground decay |
5.0 |
4 |
|
|
|
|
|
||
|
|
Below‑ground
decay |
|
5.0 |
15 |
|
|
|
|
|
|
|
|
Cattle(a) |
|
5.0 |
|
0.01 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Pasture
soil(a) |
|
5.0 |
|
|
|
0.002 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Loss
of intact |
|
5.0 |
|
0.0002 |
|
|
‑0.0004 |
‑0.004 |
|
|
|
cerrado sources(a)(b) |
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Soil
C stock |
|
5.0 |
7 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Regrowth |
|
|
5.0 |
‑9 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cerrado subtotal |
|
36 |
0.09 |
2.03 |
0.012 |
0.05 |
0.07 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
TOTAL
FOR LEGAL AMAZON |
|
|
958 |
1.42 |
35.03 |
0.165 |
‑0.74 |
1.16 |
||
|
|
|
|
|
|
|
|
|
|
|
|
|
(a) Recurring effects (cattle methane, forest soil
methane sink, pasture soil N20, hydroelectric methane) summed for 100‑year period for
consistency with IPCC 100‑year horizon calculation. |
||||||||||
|
(b)
Intact cerrado source for NOx and NMHC derived from the
forest per‑hectare emission assuming emission is proportional to the
tree leaf dry weight biomass in each ecosystem. Cerrado tree leaf
biomass (dry season) = 0.756 t/ha (dos Santos, 1989: 194). Forest (at Tucuruí, Pará) = 12.94 t/ha
(Revilla Cardenas et al., 1982: 6). |
||||||||||
|
TABLE XVI: NET COMMITTED EMISSIONS FROM 1990 DEFORESTATION WITH CO2
EQUIVALENT, 100‑YEAR TIME HORIZON |
|
|
|
||||||||||||
|
|
|
|
|
||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Low
trace gas scenario |
|
|
|
High trace gas scenario |
|
|
|
Contribution
of each gas to total effect (%) |
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Gas |
Global
warming potential(a) |
Amount
emitted (106 t of gas/year) |
|
CO2
equivalent (106 t of gas/year) |
|
Amount
emitted (106 t of gas/year) |
|
CO2
equivalent (106 t of gas/year) |
|
Low
trace gas scenario |
High
trace gas scenario |
||||
|
|
|
|
|
|
|
|
|
||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Forest |
Cerrado |
Total |
Forest |
Cerrado |
Total |
Forest |
Cerrado |
Total |
Forest |
Cerrado |
Total |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CO2 |
1 |
921.23 |
36.41 |
957.41 |
921.23 |
36.41 |
957.41 |
921.23 |
36.41 |
957.64 |
921.23 |
36.41 |
957.64 |
95.26 |
91.63 |
|
CH4 |
24.5 |
1.03 |
0.07 |
1.10 |
25.23 |
1.80 |
27.02 |
1.33 |
0.09 |
1.42 |
32.66 |
2.19 |
34.85 |
2.69 |
3.33 |
|
CO |
0 |
26.25 |
1.62 |
27.86 |
0.00 |
0.00 |
0.00 |
33.00 |
2.03 |
35.03 |
0.00 |
0.00 |
0.00 |
0.00 |
0.00 |
|
N2O |
320 |
0.06 |
0.01 |
0.06 |
18.83 |
1.83 |
20.67 |
0.15 |
0.01 |
0.16 |
48.83 |
3.83 |
52.66 |
2.06 |
5.04 |
|
NOx |
0 |
0.70 |
0.05 |
0.74 |
0.00 |
0.00 |
0.00 |
0.70 |
0.05 |
0.74 |
0.00 |
0.00 |
0.00 |
0.00 |
0.00 |
|
NMHC |
0 |
0.54 |
0.04 |
0.58 |
0.00 |
0.00 |
0.00 |
1.08 |
0.07 |
1.16 |
0.00 |
0.00 |
0.00 |
0.00 |
0.00 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
CO2‑equivalent gas (106 t) |
|
965 |
40 |
1,005 |
|
|
|
1,003 |
42 |
1,045 |
100.0 |
100.0 |
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
C02‑equivalent
carbon (106 t) |
|
263 |
11 |
274 |
|
|
|
273 |
12 |
285 |
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(a) IPCC 100‑year values expressed as kg of CO2
gas equivalent/kg of gas. The global
warming potentials are from Albritton et al., 1995: 222. |
|||||||||||||||
Fig. 1

Fig. 2

Fig. 3

Fig. 4
