The
text that follows is a PREPRINT.
Please cite as:
Nogueira, E.M., B.W. Nelson and
P.M. Fearnside. 2006. Volume and biomass of trees in central Amazonia:
Influence of irregularly shaped and hollow trunks. Forest Ecology and Management 227(1-2): 14-21.
DOI 10.1016/j foreco 2006.02.004
ISSN:
0378-1127
Copyright:
Elsevier
The
original publication is available at: http://www.elsevier.com.nl <publisher
link>
Ms. No.
FORECO1257
Volume and
biomass of trees in central Amazonia: Influence of irregularly
shaped and hollow trunks
Euler Melo Nogueiraa; Bruce Walker
Nelsonb, Philip M. Fearnsideb*
aProgram in Tropical Forest
Science, National Institute for Research in the Amazon–INPA, C.P. 478,
69.011-970 Manaus, Amazonas, Brazil.
bDepartment of
Ecology, National Institute for Research in the Amazon–INPA, C.P. 478,
69.011-970 Manaus, Amazonas, Brazil.
* Corresponding author. e-mail: pmfearn@inpa.gov.br
Revised
5 Feb. 2006
Main text 4612 words;
total 5989 words.
Abstract
Conventional
measurements of diameter, basal area and volume of the bole assume that any
cross section of the bole is circular and that the bole is a solid of
revolution. These assumptions lead to error when the bole is irregularly shaped
and/or hollow. These errors were quantified for trees in central Amazonia after
adjusting the number of trees sampled in each class based on the diameter
distribution of a large inventory. For large trees (DBH ≥ 50 cm) total
basal area was overestimated by 30%, while the overestimate was 11% for all
trees with DBH ≥ 5 cm. The total bole volume per hectare was overestimated
by 11.2% (~40 m3/ha). Most of this volume correction is attributed
to the non-circular form of the cross section; the effect of hollow areas on
the volume of the bole was only 0.7%. For trees above 31.8 cm DBH, which is the
minimum diameter in the Projeto RADAMBRASIL inventories, the volume per hectare
was overestimated by 4.4% using conventional measurements. Because of
compensating errors in commonly used formulas, however, the volume overestimate
associated with conventional methods does not imply biomass overestimation in
studies that have used the RADAMBRASIL dataset.
Keywords:
irregularities of the bole, volume of the bole, hollow trees, basal area,
biomass.
1. Introduction
Estimates
of emissions of greenhouse gases from deforestation and logging in Brazilian
Amazonia are derived from estimates of biomass obtained from large-scale
inventories of wood volume. Occurrence of irregular and hollow boles can
directly affect the estimates of volume and of greenhouse gas emissions from
the region. This is because measures of
diameter, basal area and wood volume invariably treat the bole as a solid of
revolution, making the assumption that any cross section of the bole is
circular (Ahrens and Holbert, 1981). In tropical forests, trees with indentations
and irregularly shaped boles lead to overestimates in the measures of basal
area and volume when using a measuring tape, even when diameter is measured
above any buttresses or protuberances (Clark, 2002; Clark and Clark, 2000;
Sheil, 1995). The errors are larger in species that have accentuated
irregularities in the bole, such as Aspidosperma
discolor A.DC. (Carapanaúba) and Minquartia
guianensis Aubl. (Acariquara), and in large trees, which tend to have
more irregularities and higher frequencies of hollow trunks (Clark and Clark,
2000; Fearnside, 1992, 1997a).
The occurrence of hollow trunks can also mean
significant overestimates in measurements of basal area and of real wood
volume, this being one of the uncertainties in estimates of biomass and of carbon
emissions based on forest inventories (Brown and Lugo, 1992; Fearnside, 2000).
Hollow trees are important in forest-management plans
and are associated with the presence of termites (Amelung et al., 2002) and
treefalls caused by wind and lianas (Putz et al., 1983); these affect turnover
rate, average stand age, and rates of gap formation and consequent recruitment
of pioneers. For Amazonia, estimates of hollow volume have varied from 1.6% to
9.2% of the total volume of the boles per unit of area (Fearnside, 1992, 1997b,
2000; Brown et al., 1995; Brown and Lugo, 1992).
Irregular and hollow boles will not influence biomass estimates
that are based on direct weighing of trees or on allometric equations derived from
directly weighed trees, but these characteristics of the boles will affect
biomass estimates derived from commonly used wood volume data. In this paper we evaluate the effect of
irregularities in the bole and the occurrence of hollow trunks on the measurements
diameter and basal area for trees in Amazonia. These findings have important implications for estimates of forest
volume and biomass and for carbon emissions from
deforestation.
2. Materials and Methods
2.1. Collection site
The collection areas
were six sites spread over ~45 km2 in the Tarumã Small-Farmer Rural
Settlement Project, centered about 50 km northwest of Manaus, Amazonas, Brazil
(60.16ºW; 2.83ºS). Average annual precipitation is 2075 mm, with July to
September being <100 mm/month; mean altitude is 100 m; mean monthly
temperature ranges from 26 ºC to 27.6 ºC (INMET, 2003). The vegetation is dense
upland rain forest without seasonal flooding, on nutrient-poor yellow latosol
(Oxisol)(Magnago et al., 1978; Yamazaki et al., 1978). The six sites chosen
were in primary forest, without recent natural gaps or growth of pioneers
associated with deforestation borders. The area is a new colonization front
(< 5 years old) and deforestation for agriculture was authorized by the Brazilian
Institute for the Environment and Renewable Natural Resources (IBAMA). A total
of 303 trees (DBH ≥ 5 cm) were felled in the field at random, but
following pre-defined quotas by size class. An inventory of 72 ha (Castilho, 2004) was then
used to adjust the felled sample using a distribution of tree diameters
normally found in forests (diameter classes 5-cm DBH intervals). The numbers
used to adjust the frequency of sampled trees to match that found in the forest
are based on the 72 ha inventoried by Castilho (2004) and are termed
“replication factors” in this paper (Table 1).
[Table 1 here]
2.2. Evaluation of circumference measures for obtaining
the volume of the bole.
For the 303 trees botanical samples were collected and disks of constant
thickness (~3 cm) were removed with a chainsaw. Disks were taken at breast
height and at the top of the bole, below the thickening associated with the
base of the first major branch. For 73 trees chosen randomly from the full
sample of 303 trees, two additional disks were taken, positioned at 33% and 66%
of the length between breast height and the height of the first major branch.
For all trees measurements were made of total height, height of the bole and
circumference as determined with a measuring tape at each disk-sampling point.
The measurements at breast height were made 1.36 m above the ground or above
the buttresses, if present. Each disk’s outline was drawn on a large sheet of
paper by tracing the outer edge and inner hollows if present. The drawings were
affixed to a flat wall and photographed with an 80-mm lens from a standard
distance of 4 m using a digital camera. The average pixel size in the digital
photographs was 1.876 mm on each side, determined using four reference marks on
the wall. The view angle was perpendicular and scale varied by only 0.6% from
the center to the edge of the panel.
Using
Adobe Photoshop 4.0 software (Adobe Systems, Inc.,
1996) all of the pixels were selected that corresponded to areas with
wood, using the image with amplification of 600%. The area was determined in
pixels, converted to square centimeters and compared with the cross-sectional
area obtained by the conventional method, which considers the perimeter taken
with a measuring tape in the field to be the circumference of a perfect circle
and without any hollows. Three corrected biometric attributes for each tree
(diameter, basal area and volume) were calculated from the true area of
sections of the bole, as determined by the pixel count, thereby correcting for
the effects of non-circular shape and hollow trunks. The corrected diameter
would be, for example, the diameter calculated for a perfect circle with an
area equal to that determined by the pixel count of the section cut from a
tree. Oval sections, irregularities and the presence of hollow areas mean that
the “conventional” biometric attributes will be larger than the “corrected”
attributes (Figure 1 and Table 2).
From
the corrected cross–sectional areas, the volume of each bole was calculated
using the Smalian formula: {(Asi + Asf) / 2} × h; where Asi = area of the cross
section at breast height, Asf = height at the top of the bole; and h = height
of the commercial bole. This procedure was adopted for the 73 trees for which
cross sections were sampled at four positions along the bole, and also for the
other 230 trees for which cross sections were only sampled at breast height and
near the first large branch.
2.3. Botanical identification
Herbarium
vouchers from each tree were identified by practical botanical experts
(parabotanists) who were employees of the herbarium of the National Institute
of Research in the Amazon (INPA). For the 303 trees sampled randomly, 186
different species or morpho-species were identified. Voucher specimens are kept
at INPA.
3.
Results
3.1.
Effect of non-circular form and of hollows on conventional estimates of
diameter, basal area and wood volume
For species with irregularities in
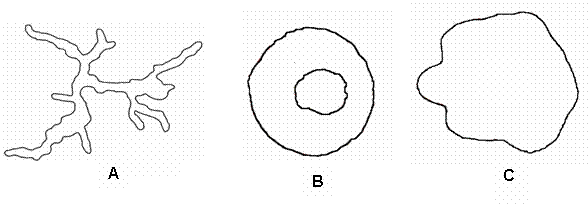
the bole, the conventional measurements of basal area can overestimate by up to
400% the real cross-sectional area of wood with bark at breast height (Figure 1
and Table 2). When the sample of 303 trees was adjusted to represent the true
frequency distribution of diameter classes in terra firme (upland) forest in central Amazonia, the corrected
diameter was, on average, 3.7% smaller at breast height and 3.1% smaller at the
top of the bole, when compared with the diameter obtained in the conventional
way (from determining the girth with a measuring tape and assuming this value
to be the circumference of a circle).
[Figure
1 and Table 2 here]
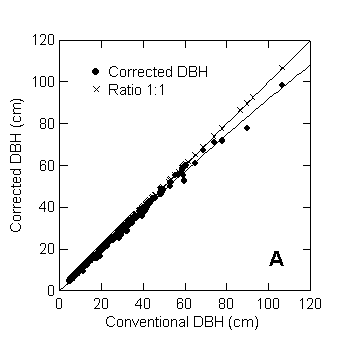
The overestimate in the diameter measures caused by the non-circular
shape and hollow trunks is greater for large trees (Figure 2a). For trees with
DBH ≥ 50 cm, adjusted to their frequency in a large inventory (i.e., “inventory-adjusted” by use of the
replication factors; see Table 1), the mean conventional DBH was 14.5% larger
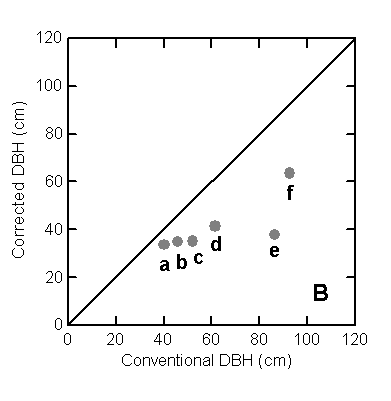
than the mean corrected DBH; about half of this effect was caused by six trees
with very irregular boles, most of which are illustrated in Figure 2b. These
six trees had conventional DBH that varied from 45 to 92 cm. At the top of the
bole, the average overestimate of the diameter was 8.6% for the same size class
(DBH ≥ 50 cm). The cross section becomes more circular in higher parts of
the bole.
[Figure 2 here]
By the conventional method, the inventory-adjusted basal area/ha was 11%
larger than the corrected basal area. The six trees with very irregular boles
were responsible for about one-third of this effect. Inventory-adjusted mean
overestimate of the area of the disk per tree was 6.6% at breast height and 6%
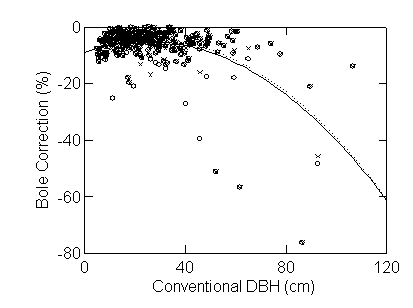
at the top of the bole (Figure 3). The error is again greater for large trees.
For trees with DBH ≥ 50 cm, the overestimate of the cross-sectional area
at breast height averaged 30%, about half of this (14%) being caused by the six
trees with very irregular boles. The median overestimate of basal area was only
12% for all trees >50 cm DBH.
[Figure 3 here]
Considering all trees with DBH ≥ 5 cm, conventional measurements
resulted in a mean overestimate per inventory-adjusted tree of 6.5% in the
volume of the bole and of 11.2% in the bole volume per hectare. This is an
overestimate of about 40 m3/ha. Again, about one-third of this (4%)
was due to the six large trees with highly irregular cross sections. For trees with DBH ≥ 50 cm (n = 23) the
inventory-adjusted overestimate of stand bole volume was of 24%, half of this
being due to the six highly irregular trees. The 23 large trees were also
responsible for almost half of the error per hectare, or approximately 24 m3/ha.
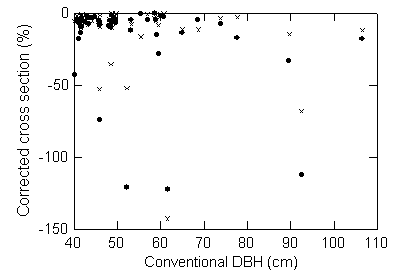
The difference in the estimate of volume was again greater for larger trees
(Figure 4). The trees with the largest overestimates of volume using
conventional measures of diameter were: Minquartia
guianensis Aubl. (common name Acariquara, DBH = 45.8 cm), 65.6%
overestimated volume; Chimarrhis
turbinata DC. (Madeira-do-Remo, DBH = 92.5 cm), 94%; Aspidosperma discolor A.DC. (Carapanaúba, DBH = 52.2 cm), 105.1%; Aspidosperma discolor A.DC (Carapanaúba, DBH = 61.6 cm), 130.6%; and Swartzia polyphylla DC. (Paracutaca, DBH
= 86.4 cm), 320.7%.
[Figure 4 here]
The volumetric estimate obtained with conventional DBH and the formula (eq.
1) used by Projeto RADAMBRASIL (1978-1983; sheet 20A, volume 18, p. 17), when
compared with the “corrected” (Smalian) volume, was 6.3% larger
(inventory-adjusted mean per tree), considering only those trees above the
minimum diameter inventoried by RADAMBRASIL (31.8 cm DBH). For volume per
hectare of trees with DBH ≥ 31.8 cm, the overestimate was only 4.4% (n =
93 prior to replication). This is much smaller than the 15.3% overestimate
found for bole volume per hectare for trees with DBH ≥ 31.8 cm calculated
by the Smalian method and conventional DBH .
V = π/4 × DBH2 × H × FF (eq.
1)
Where:
V = Volume with bark
(m3)
DBH = Diameter at
breast height (m)
H = Commercial height
(m)
FF = Form factor: 0.7.
3.2. Occurrence of hollow trees
Hollow areas
of different sizes were found in only 30 of the 303 trees sampled (Table 3).
The lowest disk (taken at 1.36 m or above any buttresses) was the most frequent
location for hollows. But at this height, only 7.7% of the sampled trees had
hollows and these, when present, occupied an average of only 9% of the
cross-sectional area. In just 3.2% of the sampled trees, hollow areas were
found at the top of the bole. Only one of the 303 trees had hollow area at both
the top of the bole and at breast height, possibly being hollow along the
entire length of the bole.
The sum of the individual basal areas of all trees indicates that the
hollow areas at breast height or just above the buttresses occupied only 1.1%
of the total cross-sectional area of the sample. At the top of the bole, the hollow area
represented 0.8% of the total area at this height for all trees. Hollows larger
than 30% of the cross-sectional area of the bole were not found at any height.
With respect to the total area of the cross section of the bole, 24 of the 303
trees had hollow areas that occupied up to 10% of the cross-sectional area; in
8 trees hollows occupied 10 to 20%, and in 5 trees the hollow area occupied 20
to 30%.
[Table
3 here]
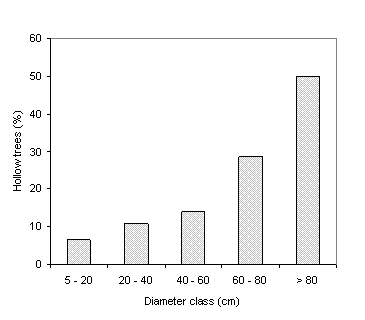
For the
sampled trees, hollow trunks were more frequent in trees with larger diameters
(Figure 5). When the occurrence of
hollow areas was ignored, volume was overestimated by only 0.6% in the 303
sampled trees and the bole volume per hectare was overestimated by 0.7% for all
trees, as compared to the volume obtained from DBH corrected for the effects of
irregularites and hollows (Smalian formula). This is because hollow areas occur
mainly in large trees, and these did not need to be replicated when adjusting
to inventory frequencies.
[Figure
5 here]
4.
Discussion
4.1. Effect of non-circular cross section and of hollow trunks on
measures of diameter, basal area and wood volume
The effects of irregularities of the bole and of buttresses on estimates
of diameter, basal area, volume and biomass have been evaluated in tropical
forests (Clark, 2002; Clark and Clark, 2000; Sheil, 1995), but there are no
studies that separate the effects buttresses and of non-circular shapes
(including those above any buttresses). Discussion in the literature has
focused on the effects of buttresses and hollows as sources of bias in measures
of biometric attributes, with possible effects on biomass estimates. Indeed,
diameter measures at breast height in trees with buttresses have been the
source of intense debate on the apparent temporal trend of increasing biomass
in permanent plots of Neotropical forests (Clark, 2002; Clark and Clark, 2000),
increasing the list of uncertainties concerning the role of tropical forests as
a source or a sink of carbon (Houghton, 2003). The occurrence of hollow trunks
has also been considered as a bias towards overestimation in biomass
calculations, up to 9% being discounted in recent estimates of carbon emissions
(Fearnside, 2000).
The results of the present study point to new considerations: (i) the
irregular form of the boles of the large trees and species with accentuated
indentations can lead to overestimation of biometric attributes, (ii) the
occurrence of hollow trunks seems to cause little error in the estimates of
wood volume. For the first effect, and at the stand level, the importance of
irregularities in the bole will be highly sensitive to the stand density of
species with very irregular boles and to the greater effect of irregularities
in large trees. Forests with high density of species with irregular boles can
contain an important bias towards overestimation. The errors caused by
irregularities and hollow trunks are more important for forest biomass than the
average bias per tree would suggest because the biases are more accentuated in
the large trees that represent a substantial amount of wood volume per hectare.
In spite of the forests of central Amazonia having less biomass in large trees,
as compared to other areas in Amazonia (see Chave et al., 2001), the percentage
reaches 23% in trees with DBH ≥ 50 cm according to an estimate of biomass
in 20 ha in central Amazonia (do Nascimento, 2002).
On the other hand, the difference between conventional and corrected
stand volume cannot be applied in a simple fashion to correct stand biomass estimates
downward. This is because allometric regressions relate conventional DBH to
true stand biomass in felled and weighed samples. Therefore, the independent
variable that must be used in biomass estimates is the conventional (not the
corrected) DBH. Biomass errors in a forest inventory can be in either
direction, depending on how the importance of irregularities and hollows in
that inventory compares with the (unfortunately unknown) importance of these
factors in the felled and weighed trees used to derive allometry.
Commercial volume is based on large trees and will be overestimated
using geometric formulas (such as equation 1). The correction factor is both
greater and more uncertain for large trees (Figures 2a, b). The error can be
minimized if the volumes of species with very irregular boles are estimated
separately. Large-scale inventories carried out in Amazonia, such as Projeto
RADAMBRASIL, included species with highly irregular boles in their volume
estimates. The Smalian formula, based on the conventional measurements of
diameter or circumference, has been applied to obtain volumes used as reference
values in developing and validating models (Ribeiro, 1996, p. 23; Moura, 1994,
p. 29; Higuchi and Ramm, 1985, p. 35; Fernandes et al., 1983, p. 539).
Naturally, the errors will be smaller in the models in which the dependent
variable was obtained using only species with boles that were more regular and
free of defects, as assumed by Fernandes et al. (1983). Such models are
appropriate for commercial wood species, but not for estimating the total
volume per unit area in a community. Species with very irregular boles also
generate significant amounts of waste when logged, and volumetric models
developed for commercial logging always result in overestimates for these
species.
4.2. Hollow trees
Hollows were more frequent and were
larger toward the base of the bole. In only one of the 303 trees was a hollow
area found at both ends of the bole. In the 73 trees sampled at four positions,
no hollows were found in the slice 2/3 of the way up the bole. However, the
number of observations was too small to make inferences about the continuity of
hollows in every bole, since hollows were found in only 10% of the trees. Considering all trees that were hollow at breast height,
among the 303 trees in the study, the total hollow area would be equivalent to
a single bole of just 25 cm diameter. The effect of hollows on bole
volume was determined by transforming trees into thinner volumes of revolution
using the true cross-sectional area of wood at the position of each disk, then
applying the Smalian formula. The effect of hollows
on the total bole volume per hectare was small: a reduction of only 0.7%. This
observation will increase the estimates of carbon emission from deforestation
in Amazonia, such as those of Fearnside (1992, 1997b, 2000), which used Projeto
RADAMBRASIL data and discounted by 6.6-9.2% for hollow boles. However, the
percentage found here is close to that reported by Brown and Lugo (1992). For
RADAMBRASIL data, these authors assumed, that hollows occupy 2% of the total
bole volume/ha, giving a 1.6% downward correction for stand biomass estimates by assuming that 20% of the boles with DBH ≥ 40 cm
are hollow up to 2 m above the ground. A similar percentage was found by Clark
and Clark (2000) in tropical forest plots in Costa Rica, where the hollow area
was estimated at 1.7% of the total volume of boles with diameter above 30 cm.
For open forests in Rondônia, Brown et al. (1995) found hollow areas in less than
20% of 53 boles examined and estimated that the total hollow volume at less
than 4%. These numbers indicate that the occurrence of hollow areas can, in
fact, represent little bias in the biomass estimates. However, it should be
pointed out that in the study in question the estimate of 0.7% hollow volume
(only in the volume of the bole) is indirect because the height of each hollow
area was not appraised along the length of the bole. For trees with DBH
over 40 cm, the percentage of hollow areas found in this study (13%) was much
smaller than the 27% reported for central Amazonia by Fearnside (1997b), based
on N. Higuchi (personal communication). For trees over 10 cm DBH (n = 145), an even higher percentage (32%) was reported by Rodrigues
and Valle (1964) for trees of sandy soil forests in central Amazonia.
4.3. Effect of non-circular and hollow trunks on the biomass estimates
The results reported here do not
affect biomass estimates that are based on direct weighing of trees or on
allometric equations derived from weighed trees. However, estimates of wood
volume in inventories could be overestimated but have this bias offset by other
variables used in the formula that is adopted for the biomass estimate. A more
detailed explanation can clarify this result.
The biomass estimates carried out starting from
volumetric data are based on forest inventories that are the only available
sources of data with broad spatial representativeness (Brown and Lugo, 1992;
Brown et al., 1989, 1991), according to the model:
TAGB = Inventoried
volume × VEF × WD × BEF (eq.
2a)
Where:
TAGB = Total above-ground biomass of standing trees ≥
10 cm DBH (Mg ha-1),
Inventoried volume = Commercial volume of the boles
above inventoried minimum DBH (m3 ha-1),
VEF = Volume expansion factor, representing the volume
of boles of trees with DBH between 10 cm and the minimum inventoried DBH,
WD = Wood density (g cm-3);
BEF = Biomass expansion factor (adds the biomass of
the crown, for all trees ≥ 10 cm DBH).
The above model has been used to obtain biomass
estimates per hectare for Brazilian Amazonia starting from RADAMBRASIL
inventories that, as demonstrated, report overestimated values of wood volume
due to irregularities and hollow trunks. However, it is necessary to evaluate
how the effects of irregularities and hollow trunks reported in the present
study will influence estimates of biomass and of carbon emissions.
The variables in equation 2a assume the following
values, according to Houghton et al. (2001), Brown (1997), Brown & Lugo
(1992) and Brown et al. (1989):
VEF = 1.25 for dense forests and 1.5 for other Amazonian forests;
WD = 0.69 g cm-3;
BEF = exp{3.213 - 0.506 Ln SB}, for SB < 190 Mg ha-1;
BEF = 1.74, for SB > 190 Mg ha-1;
SB = Stand biomass (biomass of boles ≥ 10 cm DBH) = Inventoried
volume × VEF × WD
Brown (1997, p. 6) presents another equation (eq. 2b),
which is included here for comparison with the approach of Houghton et al.
(2001):
TAGB = VOB × WD ×
BEF (eq.
2b)
Where:
TAGB = Total above-ground biomass of standing trees ≥
10 cm DBH (Mg ha-1)
WD = Wood density;
BEF = Biomass expansion factor (adds crown biomass,
for all trees ≥ 10 cm DBH),
VOB = Volume over bark of the boles of all trees ≥
10 cm DBH (m3 ha-1).
Estimates of "total above-ground biomass
(TAGB)" based on Brown and Lugo (1992), in the equations above (2a, b),
only refer to the live-tree component ≥10 cm DBH. For estimates of TAGB,
it has been suggested that the equation may result in bias due to the occurrence
of hollow trunks (Fearnside and Laurance, 2003) when using the model of Brown
and Lugo (1992), or Houghton et al. (2001). However,
in a recent publication, Fearnside and Laurance (2004) eliminate the
corrections originating from hollow areas, and conclude that the volume
estimates based on Brown et al. (1989) do not need corrections for this factor.
Corrections for irregularities in the bole are not needed when adopting the
model of Brown and Lugo (1992). First, it is necessary to review the development
of three models:
BEF = exp{3.213 -
0.506 Ln SB}, for SB < 190 Mg ha-1 (eq. 3)
BEF = 1.74, for SB
> 190 Mg ha-1; (eq.
4)
BEF = TAGB × (SB)-1 (eq.
5)
The
model in equation 5 was the basis for obtaining the coefficients presented in
equation 3 and the constant value of BEF in equation 4. It was based, in 1989,
on the “known” values of TAGB and SB for 32 plots in humid forest, almost all
in Venezuela (Brown et al., 1989). The known
values of TAGB were actually indirect estimates derived using allometric models
for individual trees, starting from D, H and WD (diameter at breast height,
total height and wood density, respectively), according to equation 6:
Dry biomass = 0.0899 (D2 × H × S)0.9522 (eq.
6)
The values of SB were also estimated using D (measured
in the field), commercial H (measured in the field), form factor of 0.7, and
WD, this last being obtained from tables for 65% to 85% of the species in the
32 inventories, or, in the case of the species with unknown WD, from the
average of WD of the known species.
Because many estimates of Amazonian biomass are ultimately
derived from the Venezuelan data (e.g.,
equation 6), the interpretation of any errors in the estimate of SB (biomass of
all boles ≥ 10 cm DBH) starting from the 32 inventories in Venezuela is
critical. TAGB in the Venezuelan data can be presumed to have been estimated
without bias, but the volume used in obtaining SB was apparently overestimated
by assuming that the bole is a perfect solid of revolution. However, this will
not create a bias in the estimate of TAGB at other sites (such as the
inventories of RADAMBRASIL in Brazilian Amazonia) because the same volume
overestimate occurs in these other inventories. BEF, based on correct TAGB and
SB overestimated in the 32 plots in Venezuela, would compensate for similar
errors in the estimate of SB in the RADAMBRASIL inventories. In other words, in
determining the BEF any consistent bias in the value of SB is compensated for
in the adjustment of the regression derived by Brown (equations 3 and 4). In
the case of the 32 plots, Brown et al. (1989) obtained BEF = 1.74 (95% CI =
0.08) from the biomasses of the individual trees in the 32 plots as estimated
using the allometric relationship for individual trees described in equation 6.
Later, Brown and collaborators considered plots with lower TAGB and obtained
equation 3 to estimate BEF starting from SB and TAGB. Therefore, there is no
reason to expect bias in the estimate of commercial volume (or SB) in the plots
studied by Brown and collaborators; since the volume (and therefore SB) of the
boles inventoried was also overestimated by RADAMBRASIL, there will not be bias
in the final estimate of TAGB when applied to the RADAMBRASIL data.
5. Conclusions
Acknowledgments
We thank the National
Council for Scientific and Technological Development (CNPq AI470765/01-1) and
the National Institute for Research in the Amazon (INPA PPI I-3620) for
financial support. Three reviewers contributed valuable comments.
6. References
Adobe
Systems, Inc. 1996. Adobe Photoshop. Version 4.0. Adobe Systems, Inc. San
Diego, California, U.S.A.
INMET, 2003. Instituto Nacional de Meteorologia. www.inmet.gov.br. Accessed 12
Aug. 2003.
Magnago, H.; Barreto, R. A. A.;
Pastore, U. 1978. Projeto RADAMBRASIL, Folha SA.20, Parte IV-Vegetação. Rio de Janeiro, Brazil. pp. 413-530.
Putz, F. E.; Coley, P. D.; Lu,
K.; Montalvo, A.; Aiello, A. 1983. Uprooting and snapping of trees: structural determinants and ecological
consequences. Canadian Journal of Forestry Research 13, 1011-1020.
Ribeiro, R. J. 1996. Estudos de função de forma para espécies
florestais de terra-firme da Amazônia. Masters dissertation in tropical
forest science, Instituto Nacional de Pesquisas da Amazônia/Fundação
Universidade do Amazonas, Manaus, Amazonas, Brazil. 76 pp.
Yamazaki, D. R.; Costa, A. M. R.; Azevedo, W.
P. 1978. Projeto
RADAMBRASIL, Folha SA.20,
Parte III-Pedologia. Rio de
Janeiro, Brazil. pp. 247-410.
FIGURE LEGENDS
Figure 1. Cross sections of boles of
trees included in Table 2: A. Swartzia polyphylla DC. (Paracutaca); B. Chrysophyllum
sanguinolentum (Pierre) Baehni ssp.
spurium (Ducke) T. D. Penn.
(Leitera), and C. Pouteria cladantha Sandwith (Abiurana).
Figure 2. A. Conventional DBH assuming a circular cross section is plotted against
the corrected DBH, which is the diameter of a circle having the true area of the
cross section determined from disk tracings. All but the six species with
highly irregular boles shown in panel B
are included. B. Irregular trees:
(a) Astronium lecointei Ducke, (b) Minquartia guianensis Aubl.,
(c) Aspidosperma discolor A. DC., (d) Aspidosperma discolor A. DC., (e) Swartzia polyphylla DC., and (f) Chimarris turbinata DC.
Figure 3. Conventional versus corrected DBH for large trees (DBH ≥ 40 cm; n
= 52). One tree was excluded for which the correction was approximately -400%.
Circle symbols represent the section at breast height (1.36 m) and “x” symbols
represent the section at the end of the bole. The plot shows clearly that the
magnitude of the correction increases at larger tree diameters and that the
relationship has high variability.
Figure 4. Estimated volume of the bole decreases when the corrected DBH is used.
The correction and the variance of the correction are both greater for larger
trees. Paired symbols for each tree show the effect of correcting only for
cross section shape (open circles) and the effect of correcting for both shape
and hollows (x symbols).
Figure
5. Frequency of hollow trees
by diameter class.
Table 1. Number of
trees sampled by size class and replication factors to emulate known stem
density in central Amazonia.
|
Conventional DBH
|
Number of trees sampled
|
Replication factor*
|
Stems/ha
|
|
≥5
- <10
|
36
|
19.8
|
714
|
|
≥10
- <15
|
45
|
5.6
|
253
|
|
≥15
- <20
|
52
|
2.6
|
136
|
|
≥20
- <25
|
41
|
1.9
|
77
|
|
≥25
- <30
|
37
|
1.3
|
47
|
|
≥30
- <35
|
27
|
1.1
|
30
|
|
≥35
- <40
|
15
|
1.5
|
22
|
|
≥40
- <45
|
17
|
1.0
|
17
|
|
≥45
- <50
|
13
|
1.0
|
13
|
|
≥50
- <55
|
3
|
2.0
|
6
|
|
≥55
- <60
|
6
|
1.0
|
6
|
|
≥60
- <65
|
3
|
1.0
|
3
|
|
≥65
- <70
|
2
|
1.0
|
2
|
|
≥70
- <75
|
1
|
1.0
|
1
|
|
≥75
- <80
|
1
|
1.0
|
1
|
|
≥80
- <85
|
0
|
-
|
0
|
|
≥85
- <90
|
2
|
1.0
|
2
|
|
≥90
- <95
|
1
|
1.0
|
1
|
|
≥95
- <100
|
0
|
-
|
0
|
|
≥100
- <105
|
0
|
-
|
0
|
|
≥105
|
1
|
1.0
|
1
|
|
Total
|
303
|
-
|
1332
|
* Replication factors are multipliers for weighting the number of trees
sampled in each DBH class by the frequency of that class in Central Amazonian
forests (i.e., the quotient of “stems/ha” divided by “number of trees
sampled”).
 Figure
5.
Figure
5.